Kluczowe dokumenty
234923
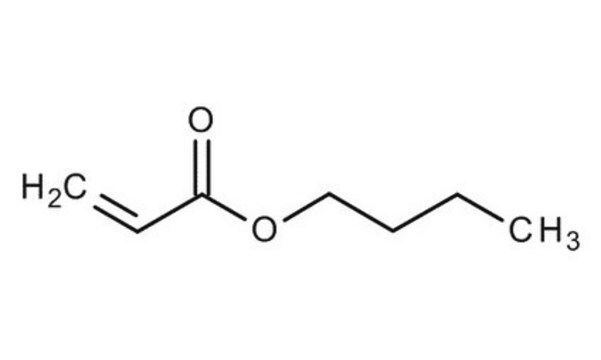
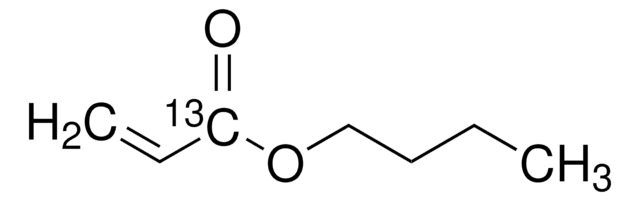
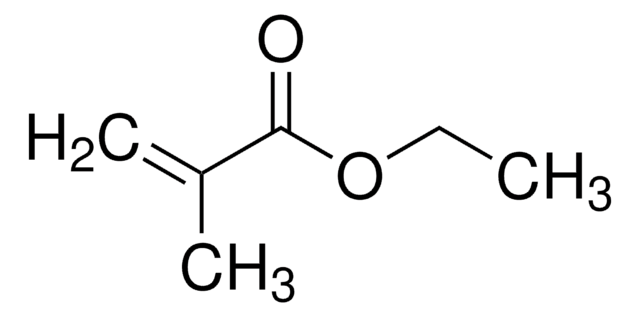
Butyl acrylate
≥99%, contains 10-60 ppm monomethyl ether hydroquinone as inhibitor
Synonim(y):
Akrylan n-butylu
About This Item
Polecane produkty
gęstość pary
>1 (vs air)
Poziom jakości
ciśnienie pary
3.3 mmHg ( 20 °C)
Próba
≥99%
Formularz
liquid
temp. samozapłonu
559 °F
zawiera
10-60 ppm monomethyl ether hydroquinone as inhibitor
granice wybuchowości
9.9 %
współczynnik refrakcji
n20/D 1.418 (lit.)
bp
145 °C (lit.)
gęstość
0.894 g/mL at 25 °C (lit.)
ciąg SMILES
CCCCOC(=O)C=C
InChI
1S/C7H12O2/c1-3-5-6-9-7(8)4-2/h4H,2-3,5-6H2,1H3
Klucz InChI
CQEYYJKEWSMYFG-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
Butyl acrylate undergoes radical copolymerization with benzoxazine containing a vinyl group to afford copolymers. Heck coupling reactions of aryl bromides with n-butyl acrylate mediated by phosphine-imidazolium salt have been reported. Copolymerization of styrene and n-butyl acrylate by ATRP catalyzed by CuBr/4,4′-di(5-nonyl)-2,2′-bipyridine has been described.
Zastosowanie
- An electrolyte additive in lithium-ion batteries to improve their low-temperature performance. The addition of BA to the electrolyte led to a significant improvement in the low-temperature performance of the battery, including enhanced ionic conductivity and improved rate capability.
- A monomer to synthesize a shape memory polymer network that contains magnetic nanoparticles for various applications, including actuators and biomedical devices.
- A monomer for the preparation of a polymeric semiconductor with intrinsically stretchable properties. This polymer material is used as a component in field-effect transistor applications.
- Poly(butyl acrylate) particles.
- Poly(butyl acrylate-b-acrylic acid) block copolymer.
- Amphiphilic charged diblock copolymers poly(butyl acrylate)-b-poly(acrylic acid).
- Poly(n-butyl acrylate), via atom transfer radical polymerization (ATRP) of n-butyl acrylate in the presence of CuIBr/4,4′-di(5-nonyl)-2,2′-bipyridine (catalyst).
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
3 - Flammable liquids
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
98.6 °F - closed cup
Temperatura zapłonu (°C)
37 °C - closed cup
Środki ochrony indywidualnej
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
The manufacture of monomers for use in ophthalmic applications is driven by the need for higher purity, improved reliability of manufacturing supply, but ultimately by the need for the increased comfort, convenience, and safety of contact lens wearers. Daily wear contact lenses have the potential to fill this need for many customers; however, their widespread use is constrained by higher costs compared to weekly- or monthly-based lenses. New approaches that improve cost structure and result in higher quality raw materials are needed to help make contact lenses more affordable and accelerate growth of the contact lens market.
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 234923-5ML | |
| 234923-1L | 4061838784810 |
| 234923-100ML | 4061838784797 |
| 234923-18L | 4061838784803 |
| 234923-25ML | |
| 234923-2.5L |
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej