추천 제품
Grade
pharmaceutical primary standard
API family
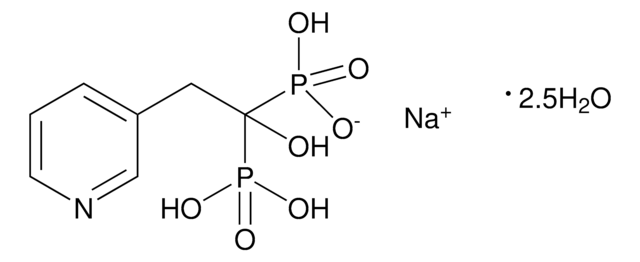
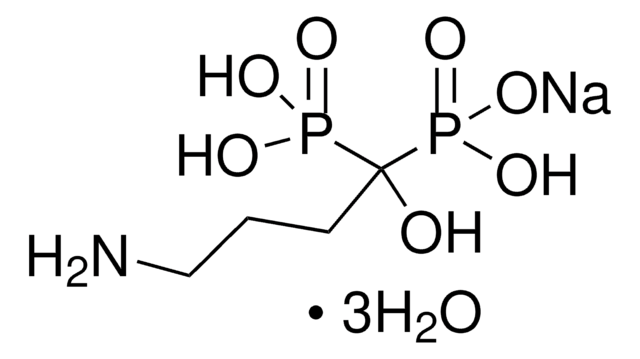
risedronate
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
OP(C(P(O)([O-])=O)(O)CC1=CC=CN=C1)(O)=O.[Na+]
InChI
1S/2C7H11NO7P2.2Na.5H2O/c2*9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6;;;;;;;/h2*1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15);;;5*1H2/q;;2*+1;;;;;/p-2
InChI key
HYFDYHPNTXOPPO-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Risedronate sodium is a member of the pyridinyl class of bisphosphonates. It is mostly used as an antiresorptive agent. It can be used in treating and preventing postmenopausal and glucocorticoid-induced osteoporosis.
애플리케이션
Risedronate sodium USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Risedronate Sodium Tablets
- Risedronate Sodium Delayed-Release Tablets
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Repr. 2 - STOT SE 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
CUNHA F.
American Journal of Surgery, 76(3), 244-260 (1948)
Eliska Vaculikova et al.
Molecules (Basel, Switzerland), 19(11), 17848-17861 (2014-11-07)
One approach for the enhancement of oral drug bioavailability is the technique of nanoparticle preparation. Risedronate sodium (Biopharmaceutical Classification System Class III) was chosen as a model compound with high water solubility and low intestinal permeability. Eighteen samples of risedronate
Yoshihisa Hirota et al.
PloS one, 10(4), e0125737-e0125737 (2015-04-16)
UbiA prenyltransferase domain-containing protein 1 (UBIAD1) plays a significant role in vitamin K2 (MK-4) synthesis. We investigated the enzymological properties of UBIAD1 using microsomal fractions from Sf9 cells expressing UBIAD1 by analysing MK-4 biosynthetic activity. With regard to UBIAD1 enzyme
Jin Woo Park et al.
Archives of pharmacal research, 37(12), 1560-1569 (2013-11-21)
Risedronate is widely used clinically to treat osteoporosis, Paget's disease, hypercalcemia, bone metastasis, and multiple myeloma. However, its oral efficacy is restricted due to its low bioavailability and severe gastrointestinal adverse effects. This study was designed to evaluate the effect
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.