1440808
USP
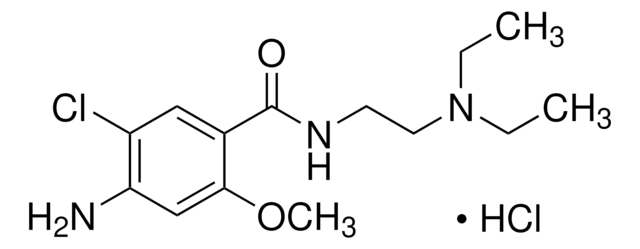
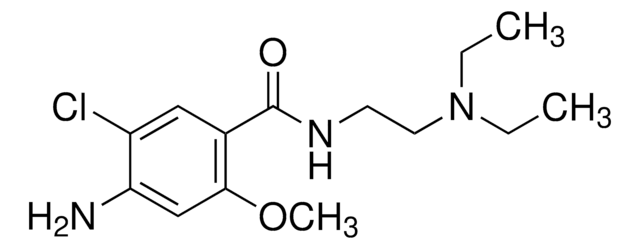
Metoclopramide hydrochloride
United States Pharmacopeia (USP) Reference Standard
동의어(들):
4-Amino-5-chloro-N-[2-(diethylamino)ethyl]-2-methoxybenzamide monohydrochloride monohydrate, Metoclopramide monohydrochloride monohydrate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C14H22ClN3O2 · HCl · H2O
CAS Number:
Molecular Weight:
354.27
MDL number:
UNSPSC 코드:
41116107
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
metoclopramide
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
[Cl-].Clc1c(cc(c(c1)C(=O)NCCN(CC)CC)OC)N.O.[H+]
InChI
1S/C14H22ClN3O2.ClH.H2O/c1-4-18(5-2)7-6-17-14(19)10-8-11(15)12(16)9-13(10)20-3;;/h8-9H,4-7,16H2,1-3H3,(H,17,19);1H;1H2
InChI key
KJBLQGHJOCAOJP-UHFFFAOYSA-N
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Metoclopramide hydrochloride USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
Also, for use with USP monographs such as:
Also, for use with USP monographs such as:
- Metoclopramide Tablets
- Metoclopramide Oral Solution
- Metoclopramide Injection
- Prazosin Hydrochloride
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Diana Egerton-Warburton et al.
Emergency medicine Australasia : EMA, 25(3), 207-212 (2013-06-14)
The study aims to determine if slow intravenous infusion of metoclopramide reduces the incidence of acute drug-induced akathisia (DIA) compared with intravenous bolus. A prospective, double-blind, double dummy trial of adult patients requiring intravenous metoclopramide in the ED. Participants were
Jian-qin Lv et al.
Trials, 14, 153-153 (2013-05-29)
The incidence of postoperative nausea and vomiting (PONV) is 50 to 79% after neurosurgery. Our study is designed to evaluate the efficacy of pericardium 6 (P6; also known as Neiguan) acupoint stimulation versus placebo, and versus routine antiemetic for the
Anastasios Koulaouzidis et al.
Current medical research and opinion, 29(9), 1171-1185 (2013-06-25)
The use of purging for bowel cleansing prior to small-bowel capsule endoscopy (SBCE) has now been established in clinical practice. Despite that, the number of incomplete SBCEs is still around 15-20%. To date, the use of prokinetics in SBCE -
Ray M Merrill et al.
BMC psychiatry, 13, 152-152 (2013-05-30)
To identify the incidence rate of spontaneous dyskinesia (SD) and tardive dyskinesia (TD) in a general population and to examine the association between dykinesia and potential risk factors (exposure to metoclopramide [MCP], antipsychotic drugs, and history of diabetes and psychoses).
Benjamin W Friedman et al.
Neurology, 82(11), 976-983 (2014-02-14)
We compared the efficacy of IV valproate with metoclopramide and with ketorolac in patients presenting to an emergency department (ED) with acute migraine. This was a double-blind comparative efficacy trial. Patients were randomized to 1,000 mg sodium valproate, 10 mg
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![[des-Gly10, D-Ala6]-LH-RH ethylamide acetate salt hydrate ≥97% (HPLC), powder](/deepweb/assets/sigmaaldrich/product/structures/150/195/13e08743-1592-4a6b-937d-559f571a2193/640/13e08743-1592-4a6b-937d-559f571a2193.png)