1327000
USP
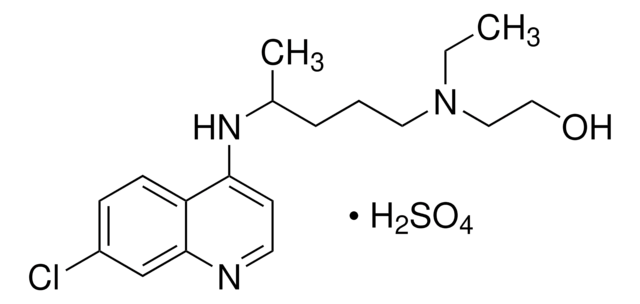
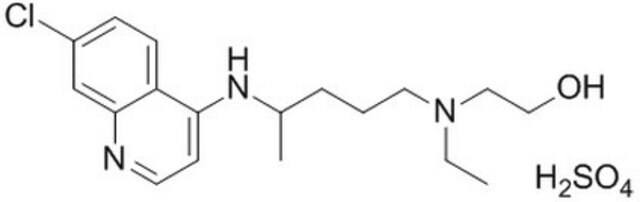
Hydroxychloroquine sulfate
United States Pharmacopeia (USP) Reference Standard
동의어(들):
Hydroxychloroquine sulfate, 7-Chloro-4-[4-(N-ethyl-N-b-hydroxyethylamino)-1-methylbutylamino]quinoline sulfate
About This Item
추천 제품
생물학적 소스
synthetic
Grade
pharmaceutical primary standard
Agency
USP
API family
hydroxychloroquine
포장
pkg of 200 mg
제조업체/상표
USP
저장 조건
protect from light
색상
white to off-white
pH
3.5-5.5 (1% in aqueous solution, pH <6.0)
mp
388.4 °F (198°C; 240°C)
solubility
chloroform: practically insoluble
ethanol: practically insoluble
ether: practically insoluble
water: soluble
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
OS(O)(=O)=O.CCN(CCO)CCCC(C)Nc1ccnc2cc(Cl)ccc12
InChI
1S/C18H26ClN3O.H2O4S/c1-3-22(11-12-23)10-4-5-14(2)21-17-8-9-20-18-13-15(19)6-7-16(17)18;1-5(2,3)4/h6-9,13-14,23H,3-5,10-12H2,1-2H3,(H,20,21);(H2,1,2,3,4)
InChI key
JCBIVZZPXRZKTI-UHFFFAOYSA-N
유전자 정보
human ... TLR7(51284) , TLR9(54106)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Hydroxychloroquine Sulfate is a salt of hydroxychloroquine (HCQ, Plaquenil), a 4-aminoquinoline based antiviral drug.
애플리케이션
- Hydroxychloroquine Sulfate Tablets
- Hydroxychloroquine Sulfate Compounded Oral Suspension
- Chloroquine Phosphate
생화학적/생리학적 작용
분석 메모
기타 정보
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
프로토콜
Under applied conditions, system suitability criteria are met, and the Chloroquine Phosphate HPLC Assay and Impurity Profiling Methods demonstrate good resolution/selectivity, reproducibility, and sensitivity.
Under applied conditions, system suitability criteria are met, and the Chloroquine Phosphate HPLC Assay and Impurity Profiling Methods demonstrate good resolution/selectivity, reproducibility, and sensitivity.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.