1046205
USP
Aztreonam
United States Pharmacopeia (USP) Reference Standard
동의어(들):
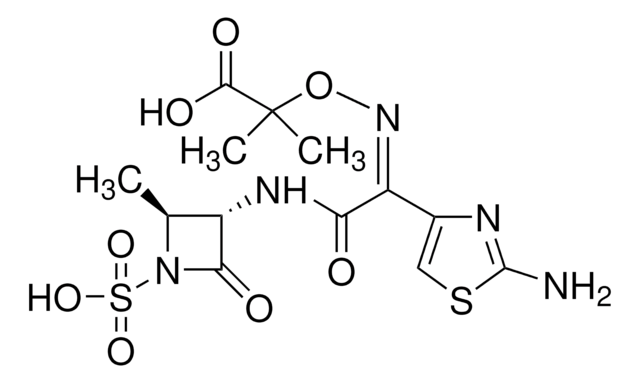
[2S-[2α,3β(Z)]]-2-[[[1-(2-Amino-4-thiazolyl)-2-[(2-methyl-4-oxo-1-sulfo-3-azetidinyl)amino]-2-oxoethylidene]amino]oxy]-2-methylpropanoic acid
About This Item
추천 제품
Grade
pharmaceutical primary standard
API family
aztreonam
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
−20°C
SMILES string
C[C@H]1[C@H](NC(=O)\C(=N/OC(C)(C)C(O)=O)c2csc(N)n2)C(=O)N1S(O)(=O)=O
InChI
1S/C13H17N5O8S2/c1-5-7(10(20)18(5)28(23,24)25)16-9(19)8(6-4-27-12(14)15-6)17-26-13(2,3)11(21)22/h4-5,7H,1-3H3,(H2,14,15)(H,16,19)(H,21,22)(H,23,24,25)/b17-8-/t5-,7-/m0/s1
InChI key
WZPBZJONDBGPKJ-VEHQQRBSSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
Also used to prepare standard and system suitability solutions for assay according to the given below monographs of United States Pharmacopeia (USP):
- Aztreonam
- Aztreonam for Injection
- Aztreonam Injection
분석 메모
기타 정보
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.