SML2416
HET0016
≥95% (HPLC)
동의어(들):
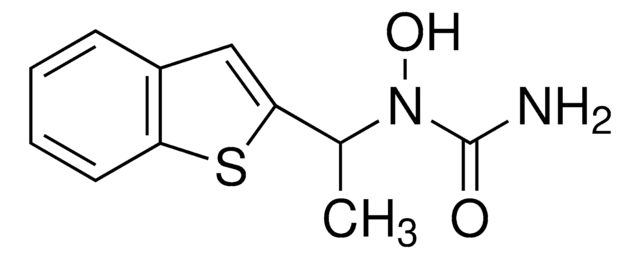
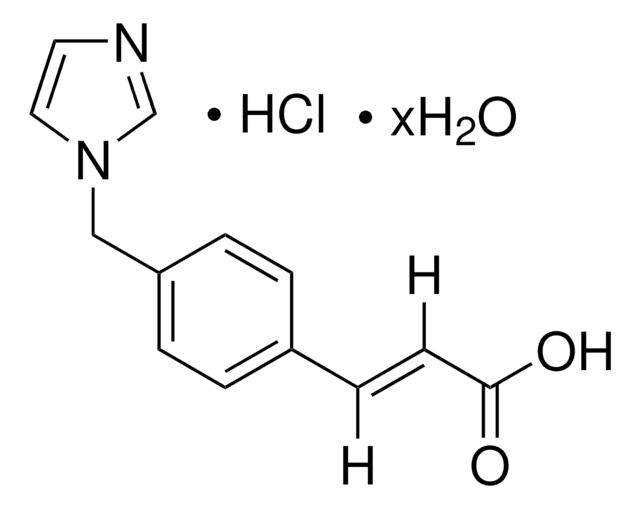
HET 0016, HET-0016, N′-(4-Butyl-2-methylphenyl)-N-hydroxymethanimidamide, N-(4-Butyl-2-methylphenyl)-N′-hydroxyformamidine, N-(4-Butyl-2-methylphenyl)-N′-hydroxymethanimidamide, N-Hydroxy-N′-(4-n-butyl-2-methylphenyl)formamidine
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
추천 제품
분석
≥95% (HPLC)
형태
powder
저장 조건
desiccated
색상
white to beige
solubility
DMSO: 2 mg/mL, clear
저장 온도
−20°C
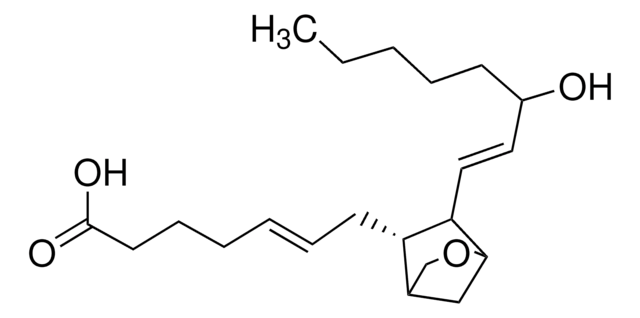
SMILES string
CCCCC1=CC=C(C(C)=C1)/N=C/NO
InChI
1S/C12H18N2O/c1-3-4-5-11-6-7-12(10(2)8-11)13-9-14-15/h6-9,15H,3-5H2,1-2H3,(H,13,14)
InChI key
LYNOGBKNFIHKLE-UHFFFAOYSA-N
생화학적/생리학적 작용
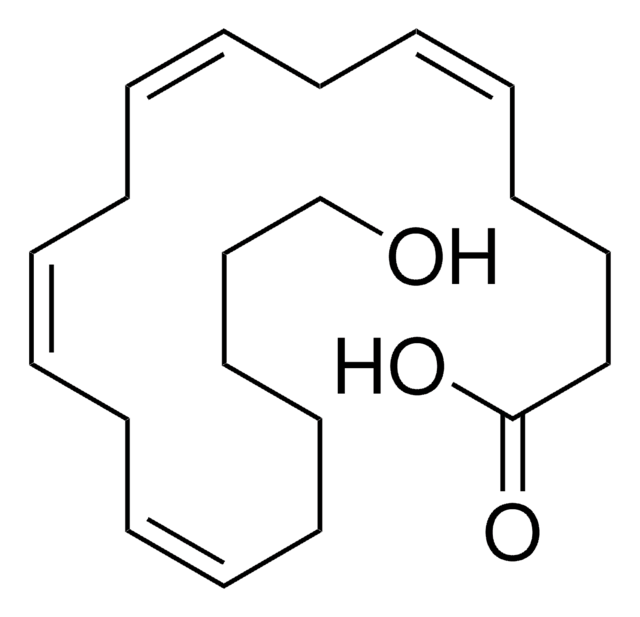
HET0016 is a potent and selective inhibitor against cytochrome P450 (CYP450) ω-hydroxylases (human CYP4A11/4A22/4F2/4F3, rat CYP4A1/4A2/4A3/4A8)-mediated 20-hydroxyeicosatetraenoic acid (20-HETE) biosynthesis (IC50 = 8.9/35 nM using human/rat renal microsomes), exhibiting much reduced potency against epoxyeicosatrienoic acids (EETs) biosynthesis (IC50 = 2.8 μM/rat microsome) or the enzyme activities of CYP2C9/2D6/3A4 and COX (IC50 = 3.3/83.9/71 μM and 2.3 μM). HET0016 is widely employed both in cultures (1-10 μM) and in aminals in vivo (1-10 mg/kg via i.v., i.m., i.p. or s.c.) for probing 20-HETE-dependent physiological and pathological processes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
N Miyata et al.
British journal of pharmacology, 133(3), 325-329 (2001-05-26)
The present study examined the inhibitory effects of N-hydroxy-N'-(4-butyl-2-methylphenyl)-formamidine (HET0016) on the renal metabolism of arachidonic acid by cytochrome P450 (CYP) enzymes. HET0016 exhibited a high degree of selectivity in inhibiting the formation of 20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) in rat renal
Fan Fan et al.
Frontiers in bioscience (Landmark edition), 21, 1427-1463 (2016-04-23)
Cytochrome P450s enzymes catalyze the metabolism of arachidonic acid to epoxyeicosatrienoic acids (EETs), dihydroxyeicosatetraenoic acid and hydroxyeicosatetraeonic acid (HETEs). 20-HETE is a vasoconstrictor that depolarizes vascular smooth muscle cells by blocking K+ channels. EETs serve as endothelial derived hyperpolarizing factors.
Xiaoning Han et al.
Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 271678X18762645-271678X18762645 (2018-02-28)
20-HETE, an arachidonic acid metabolite synthesized by cytochrome P450 4A, plays an important role in acute brain damage from ischemic stroke or subarachnoid hemorrhage. We tested the hypothesis that 20-HETE inhibition has a protective effect after intracerebral hemorrhage (ICH) and
Manoocher Soleimani et al.
PloS one, 11(7), e0159804-e0159804 (2016-07-22)
Contribution of salt wasting and volume depletion to the pathogenesis of hypercalciuria and hyperphosphaturia is poorly understood. Pendrin/NCC double KO (pendrin/NCC-dKO) mice display severe salt wasting under basal conditions and develop profound volume depletion, prerenal renal failure, and metabolic alkalosis
Guangrui Lai et al.
Prostaglandins & other lipid mediators, 134, 123-130 (2017-08-16)
We previously found that 20-hydroxyeicosatetraeonic acid (20-HETE) showed an effect on proteasome activity in cytochrome P450 F2 (CYP4F2) transgenic mice. Proteasome subunit β5 (PSMB5) is a primary subunit of the proteasome. In the current study, we examine whether 20-HETE has
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.