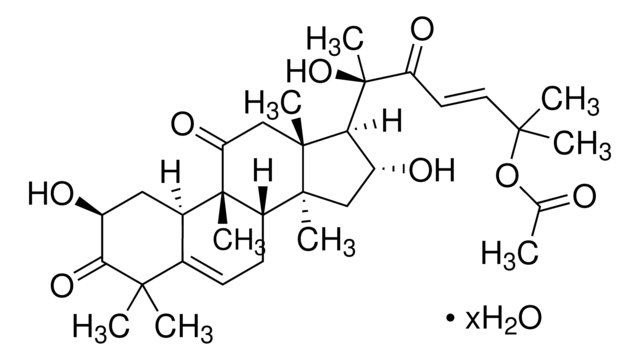
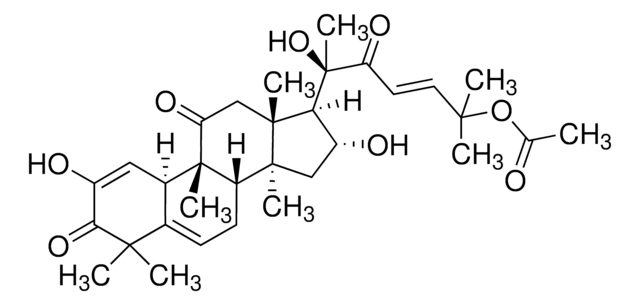
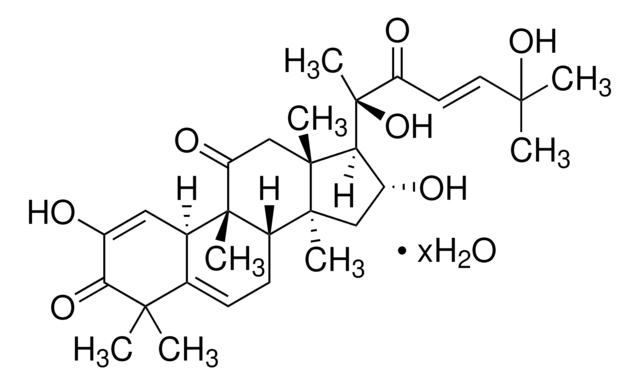
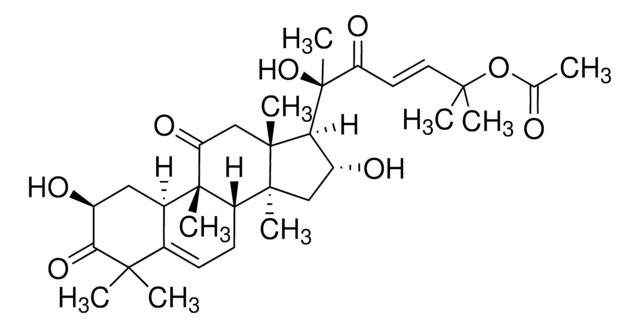
추천 제품
Quality Level
분석
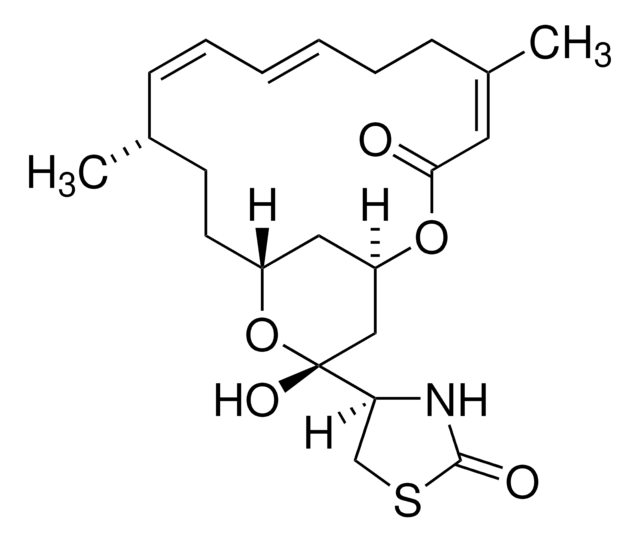
≥95% (HPLC)
양식
powder
광학 활성
[α]/D -60 to -75°, c = 0.7 (CDCl3)
색상
white to beige
solubility
DMSO: 15 mg/mL, clear
저장 온도
−20°C
InChI
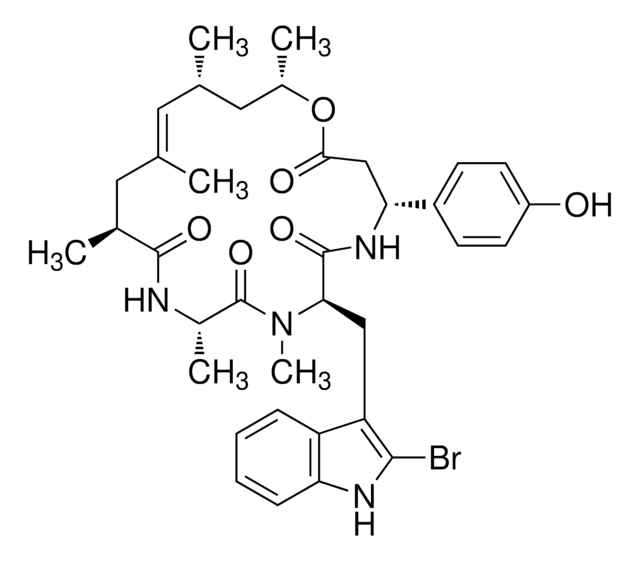
1S/C32H44O8/c1-17(33)40-27(2,3)13-12-23(36)32(9,39)25-21(35)15-29(6)22-11-10-18-19(14-20(34)26(38)28(18,4)5)31(22,8)24(37)16-30(25,29)7/h10,12-14,19,21-22,25,34-35,39H,11,15-16H2,1-9H3/b13-12+/t19-,21-,22+,25+,29+,30-,31+,32+/m1/s1
InChI key
NDYMQXYDSVBNLL-MUYMLXPFSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Cucurbitacin E has been used as a cofilin inhibitor. It is also used as a F-actin stabilizer to prevent membrane-associated periodic skeleton (MPS) loss and protect from axonal fragmentation.
생화학적/생리학적 작용
Cucurbitacin E is a potent inhibitor of actin depolymerization. Cucurbitacin E is more active than jasplakinolide, and has a different mechanism of action, binding to a different site. Cucurbitacin E binds specifically to filamentous actin (F-actin) forming a covalent bond at residue Cys257, but not to monomeric actin (G-actin), stabilizing F-actin, without affecting actin polymerization or nucleation.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Pei-Lin Wu et al.
Chemical & pharmaceutical bulletin, 52(3), 345-349 (2004-03-03)
Three new compounds: begonanline (1). nantoamide (2). and methyl (S)-glycerate (3). as well as forty-four known compounds have been isolated and characterized from the rhizomes of Begonia nantoensis. The structures of these compounds were determined by spectral analyses and/or X-ray
Manali Dimri et al.
Development (Cambridge, England), 144(14), 2595-2605 (2017-07-20)
The intrahepatic biliary network is a highly branched three-dimensional network lined by biliary epithelial cells, but how its branching patterns are precisely established is not clear. We designed a new computer-based algorithm that quantitatively computes the structural differences of the
Yasuyuki Sadzuka et al.
International journal of pharmaceutics, 354(1-2), 63-69 (2007-12-07)
We screened various food components for their ability to inhibit doxorubicin (DOX) permeability in tumor cells in vitro with the aim of finding novel modulators. Capsaicin did not change DOX permeability in the tumor cells, although the capsaicin derivatives gingerol
Tehila Tannin-Spitz et al.
Biochemical and biophysical research communications, 364(1), 181-186 (2007-10-19)
The cucurbitacins are of great interest because of the wide range of biological activities they exhibit in plants and animals. We studied the antioxidant properties of cucurbitacin B + E glucosides (cucurbitacin glucoside combination, CGC) and their direct free-radical scavenging
Yanmin Dong et al.
Carcinogenesis, 31(12), 2097-2104 (2010-08-25)
Cucurbitacin E (CuE, α-elaterin), a tetracyclic triterpenes compound from folk traditional Chinese medicine plants, has been shown to inhibit cancer cell growth, inflammatory response and bilirubin-albumin binding. However, the effects of CuE on tumor angiogenesis and its potential molecular mechanism
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.