추천 제품
분석
≥98% (HPLC)
양식
powder
광학 활성
[α]/D +30 to +40°, c = 1 in H2O
색상
white to beige
solubility
H2O: 10 mg/mL, clear
저장 온도
room temp
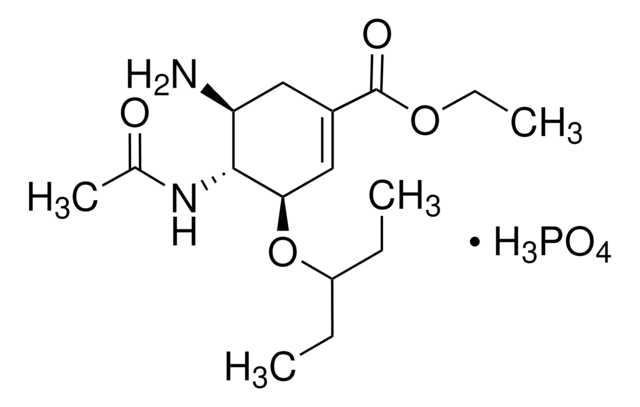
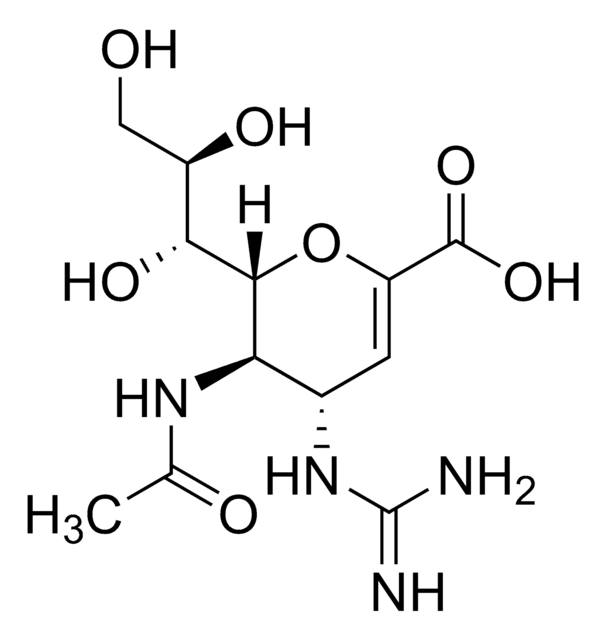
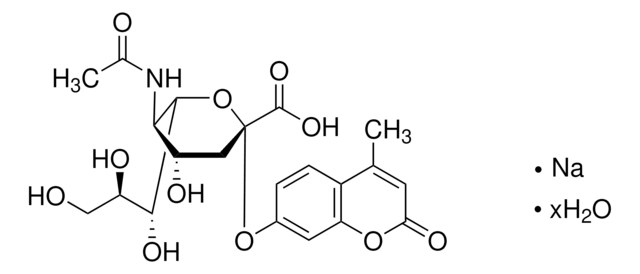
SMILES string
CC(=O)N[C@@H]1[C@@H](NC(N)=N)C=C(O[C@H]1[C@H](O)[C@H](O)CO)C(O)=O
InChI
1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1
InChI key
ARAIBEBZBOPLMB-UFGQHTETSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Zanamivir has been used:
- as a neuraminidase inhibitor to study its effects on influenza A virus (IAV) like particles
- as a neuraminidase inhibitor to study its effects on lipopolysaccharide (LPS)-mediated pathology in sepsis
- as an antiviral to study its effects on influenza B virus by plaque reduction assay
생화학적/생리학적 작용
Zanamivir exhibits therapeutic effects against influenza caused by influenza virus A and B.
Zanamivir is an influenza viral neuraminidase inhibitor.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Zanamivir
Waghorn S L and Goa K L
Drugs, 55(5), 721-725 (1998)
Emi Takashita et al.
Antimicrobial agents and chemotherapy, 59(5), 2607-2617 (2015-02-19)
Between September 2013 and July 2014, 2,482 influenza 2009 pandemic A(H1N1) [A(H1N1)pdm09] viruses were screened in Japan for the H275Y substitution in their neuraminidase (NA) protein, which confers cross-resistance to oseltamivir and peramivir. We found that a large cluster of
Replication-Competent Influenza B Reporter Viruses as Tools for Screening Antivirals and Antibodies.
Benjamin O Fulton et al.
Journal of virology, 89(23), 12226-12231 (2015-09-25)
Influenza B virus is a human pathogen responsible for significant health and economic burden. Research into this pathogen has been limited by the lack of reporter viruses. Here we describe the development of both a replication-competent fluorescent influenza B reporter
Carl J Heneghan et al.
BMJ (Clinical research ed.), 348, g2547-g2547 (2014-05-09)
To describe the potential benefits and harms of zanamivir. Systematic review of clinical study reports of randomised placebo controlled trials and regulatory information Clinical study reports, trial registries, electronic databases, regulatory archives, and correspondence with manufacturers. Randomised placebo controlled trials
Synthetic approaches to the neuraminidase inhibitors zanamivir (Relenza) and oseltamivir phosphate (Tamiflu) for the treatment of influenza.
Javier Magano
Chemical reviews, 109(9), 4398-4438 (2009-06-23)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.