추천 제품
제품명
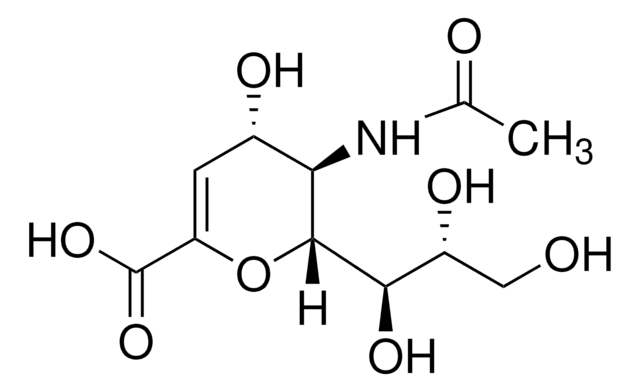
Siastatin B, lyophilized powder, from microbial
생물학적 소스
microbial
Quality Level
양식
lyophilized powder
포장
vial of 23.0 μmol
solubility
H2O: 2.3 mL/vial (for a 10 mM solution)
저장 온도
−20°C
SMILES string
CC(=O)N[C@H]1NC[C@@H]([C@H](O)[C@@H]1O)C(O)=O
InChI
1S/C8H14N2O5/c1-3(11)10-7-6(13)5(12)4(2-9-7)8(14)15/h4-7,9,12-13H,2H2,1H3,(H,10,11)(H,14,15)/t4-,5-,6-,7+/m0/s1
InChI key
DQTKLICLJUKNCG-ZTYPAOSTSA-N
애플리케이션
Broad spectrum inhibitor of sialidase.
생화학적/생리학적 작용
Streptomyces metabolite, a broad-spectrum inhibitor of neuraminidase (sialidase).
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
T Takatsu et al.
The Journal of antibiotics, 49(1), 54-60 (1996-01-01)
Novel heparanse inhibitors, A72363 A-1, A-2, and C, were isolated from the culture filtrate of Streptomyces nobilis SANK 60192 by column chromatography on various resinous adsorbents, followed by preparative anion exchange HPLC. Spectroscopic studies revealed that they are diastereomers of
K I Kondo et al.
Natural product letters, 15(6), 371-375 (2002-02-13)
N-Alkyl-3-decarboxy-3-hydroxymethylsiastatin B, N-alkyl analogues of gem-diamine 1-N-iminosugars, is a new family of glycosidase inhibitors that have been synthesized from siastatin B isolated from Streptomyces culture. These compounds were evaluated as glycosidase inhibitors.
Y Nishimura et al.
Bioorganic & medicinal chemistry, 4(1), 91-96 (1996-01-01)
N-Acetylgalactosamine-based 1-N-iminosugars, new types of glycosidase inhibitor have been synthesized by modeling on siastatin B, isolated from a Streptomyces culture. The analogues of siastatin B were proved to be potent inhibitors for alpha-N-acetylgalactosaminidase and/or beta-N-acetylglucosaminidase.
Synthesis and antimetastatic activity of 6-trichloroacetamido and 6-guanidino analogues of siastatin B.
T Satoh et al.
The Journal of antibiotics, 49(3), 321-325 (1996-03-01)
T Kudo et al.
The Journal of antibiotics, 45(6), 954-962 (1992-06-01)
Totally synthetic analogues of siastatin B, optically active 2-acetamido-3,4,5-trihydroxypiperidines having the nitromethyl, aminomethyl and carboxyl branched groups at C-5 have been obtained from D-ribono-1,4-lactone by a stereospecific convergent method. Some analogues showed inhibitory activity against some glycosidases.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.