추천 제품
분석
≥90% (HPLC)
양식
powder
저장 조건
desiccated
색상
white to beige
solubility
DMSO: >5 mg/mL
저장 온도
2-8°C
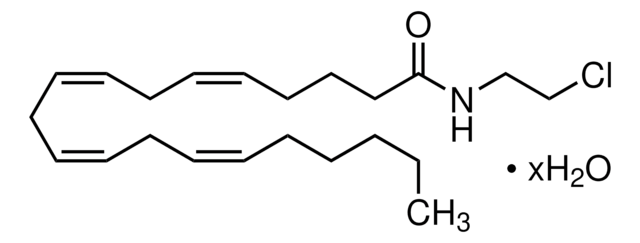
SMILES string
COc1ccc(cc1)C(=O)c2c(C)n(CCN3CCOCC3)c4cc(I)ccc24
InChI
1S/C23H25IN2O3/c1-16-22(23(27)17-3-6-19(28-2)7-4-17)20-8-5-18(24)15-21(20)26(16)10-9-25-11-13-29-14-12-25/h3-8,15H,9-14H2,1-2H3
InChI key
JHOTYHDSLIUKCJ-UHFFFAOYSA-N
애플리케이션
AM630 has been used:
- as a cannabinoid 2(CB2) inhibitor to study the analgesic effect exerted by polysaccharopeptide from Trametes versicolor (TPSP).
- as a CB2 antagonist along with β-caryophyllene (BCP) to study its effects on re-epithilialization of fibroblast cells.
- as a CB2 antagonist to study its interaction with 17-β-estradiol in primary human osteoblasts.
생화학적/생리학적 작용
AM630 is a selective CB2 cannabinoid antagonist/inverse agonist (Ki = 31.2 nM) with > 150-fold selectivity over CB1 receptors.
AM630 is a selective CB2 cannabinoid antagonist/inverse agonist.
AM630 is an aminoalkylindole and acts as a competitive antagonist of CP 55,940 and WIN 55,212-2. It also behaves as a competitive antagonist of anandamide and (R)-(+)-arachidonyl-1′-hydroxy-2′-propylamide (AM356).
특징 및 장점
This compound is featured on the Cannabinoid Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Francesca Rossi et al.
The Journal of clinical endocrinology and metabolism, 101(9), 3469-3478 (2016-06-14)
Obesity is associated with a low-grade inflammatory state and adipocyte (ADP) hyperplasia/hypertrophy. Obesity inhibits the "browning" of white adipose tissue. Cannabinoid receptor 2 (CB2) agonists reduce food intake and induce antiobesity effect in mice. A common missense CB2 variant, Q63R
Massimo Nabissi et al.
Oncotarget, 7(47), 77543-77557 (2016-10-22)
Several studies showed a potential anti-tumor role for cannabinoids, by modulating cell signaling pathways involved in cancer cell proliferation, chemo-resistance and migration. Cannabidiol (CBD) was previously noted in multiple myeloma (MM), both alone and in synergy with the proteasome inhibitor
Sachiko Koyama et al.
PloS one, 14(12), e0216104-e0216104 (2019-12-17)
Beta-caryophyllene is an odoriferous bicyclic sesquiterpene found in various herbs and spices. Recently, it was found that beta-caryophyllene is a ligand of the cannabinoid receptor 2 (CB2). Activation of CB2 will decrease pain, a major signal for inflammatory responses. We
K A Jenkin et al.
British journal of pharmacology, 173(7), 1128-1142 (2014-12-30)
In diabetic nephropathy agonism of CB2 receptors reduces albuminuria and podocyte loss; however, the role of CB2 receptors in obesity-related nephropathy is unknown. The aim of this study was to determine the role of CB2 receptors in a model of
Marko Hojnik et al.
Biomedical reports, 3(4), 554-558 (2015-07-15)
The bone remodeling process is influenced by various factors, including estrogens and transmitters of the endocannabinoid system. In osteoblasts, cannabinoid receptors 2 (CB-2) are expressed at a much higher level compared to CB-1 receptors. Previous studies have shown that estrogens
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.