N2127
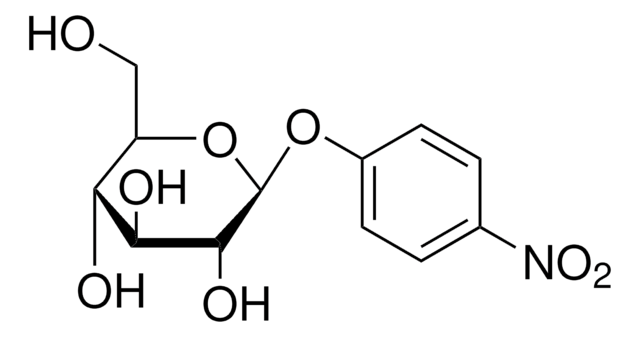
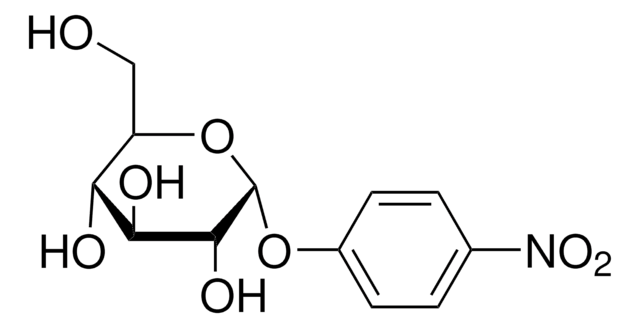
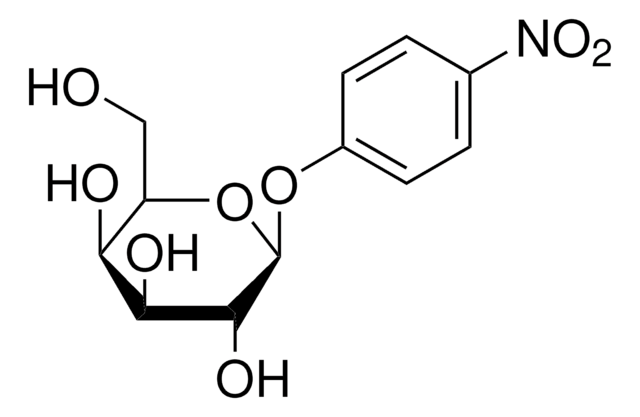
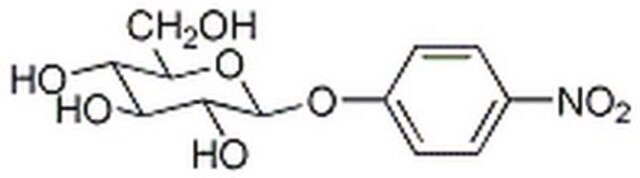
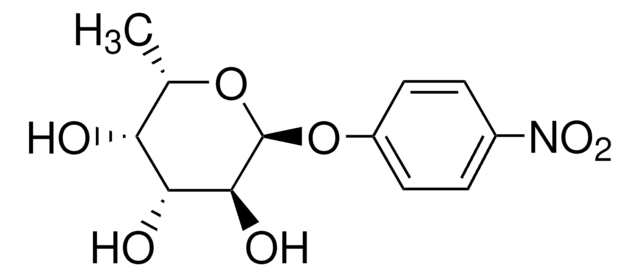
4-Nitrophenyl α-D-mannopyranoside
α-mannosidase substrate, chromogenic, ≥98% (TLC), powder
동의어(들):
4-Nitrophenyl a-D-mannopyranoside, 4-Nitrophenyl alpha-D-mannopyranoside, pNP-alpha-D-Man, pNPalphaMan
로그인조직 및 계약 가격 보기
모든 사진(6)
About This Item
실험식(Hill 표기법):
C12H15NO8
CAS Number:
Molecular Weight:
301.25
Beilstein:
92210
EC Number:
MDL number:
UNSPSC 코드:
12352204
PubChem Substance ID:
NACRES:
NA.32
추천 제품
product name
4-Nitrophenyl α-D-mannopyranoside, α-mannosidase substrate
Quality Level
분석
≥98% (TLC)
형태
powder
solubility
DMF: 50 mg/mL
저장 온도
−20°C
SMILES string
OC[C@H]1O[C@H](Oc2ccc(cc2)[N+]([O-])=O)[C@@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C12H15NO8/c14-5-8-9(15)10(16)11(17)12(21-8)20-7-3-1-6(2-4-7)13(18)19/h1-4,8-12,14-17H,5H2/t8-,9-,10+,11+,12+/m1/s1
InChI key
IFBHRQDFSNCLOZ-GCHJQGSQSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
4-Nitrophenyl α-D-mannopyranoside is a chromogenic substrate for α-mannosidase.
애플리케이션
4-Nitrophenyl α-D-mannopyranoside has been used as a substrate for α-mannosidase in hydrolase activity assay.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
S Howard et al.
Biochemical and biophysical research communications, 238(3), 896-898 (1997-10-23)
The stereochemical course of the hydrolyses catalysed by two alpha-mannosidases has been determined directly by 1H NMR. Synthetic substrates were incubated with the enzymes and the anomeric configuration of the initially formed product was ascertained in each case by observation
S J Hamodrakas et al.
International journal of biological macromolecules, 11(1), 17-22 (1989-02-01)
Molecular models for the complex formed between the lectin concanavalin A (Con A) and the saccharide derivative 4'-nitrophenyl-alpha-D-mannopyranoside (alpha-PNM) are presented, combining evidence from 1H-n.m.r. measurements, semi-empirical energy calculations and interactive graphics modelling. The models are in good agreement with
S Jelinek-Kelly et al.
The Journal of biological chemistry, 260(4), 2253-2257 (1985-02-25)
Fractionation of a crude extract from Saccharomyces cerevisiae X-2180 on Sepharose 6B in the presence of 0.5% Triton X-100 resolves two enzyme fractions containing alpha-mannosidase activity. Fraction I which is excluded from the gel contains alpha-mannosidase activity toward both p-nitrophenyl-alpha-D-mannopyranoside
Y Ohyama et al.
The Journal of biological chemistry, 260(11), 6882-6887 (1985-06-10)
The interactions of Sepharose 4B-immobilized concanavalin A (ConA) with 10 glycoasparagines derived from ovalbumin were investigated quantitatively by frontal affinity chromatography. In this method, a carbohydrate solution is applied continuously to a ConA-Sepharose column and the retardation of the elution
I Sielezneff et al.
Chirurgie; memoires de l'Academie de chirurgie, 124(2), 159-164 (1999-06-01)
Peritoneal colonization is a crucial event in the pathogenesis of peritonitis and its local complications. Adherence to the serosal mesothelium is mediated in a number of microorganisms derived from the digestive tract (especially E. coli) by type-1 fimbriae which have
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.