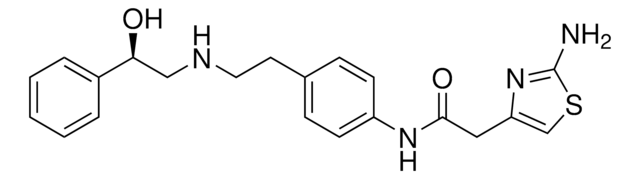
E6910
Pioglitazone hydrochloride
≥98% (HPLC), powder, hepatic gluconeogenesis blocker
동의어(들):
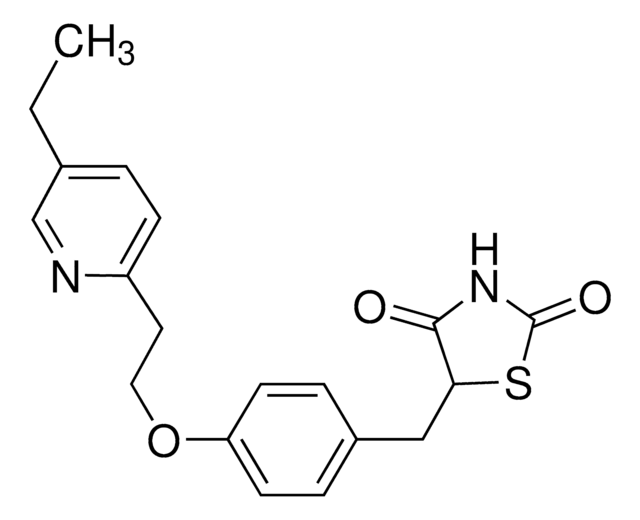
5-[[4-[2-(5-Ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione monohydrochloride
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C19H20N2O3S · HCl
CAS Number:
Molecular Weight:
392.90
MDL number:
UNSPSC 코드:
41106305
PubChem Substance ID:
NACRES:
NA.77
추천 제품
제품명
Pioglitazone hydrochloride, ≥98% (HPLC)
분석
≥98% (HPLC)
양식
powder
색상
white to off-white
solubility
DMSO: ≥10 mg/mL
주관자
Takeda
저장 온도
room temp
SMILES string
Cl.CCc1ccc(CCOc2ccc(CC3SC(=O)NC3=O)cc2)nc1
InChI
1S/C19H20N2O3S.ClH/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17;/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23);1H
InChI key
GHUUBYQTCDQWRA-UHFFFAOYSA-N
유전자 정보
human ... PPARG(5468)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Pioglitazone hydrochloride consists of poly-morphs, form I and form II. It is an oral antidiabetic agent, that is a member of the thiazolidinedione group.
애플리케이션
Pioglitazone hydrochloride has been used:
- to administer to mice model and treated the hepatoma cell line to study its effect on regulating insulin-degrading enzyme (IDE) in diet-induced obese (DIO) C57BL/6 mice
- in drug preparation to analyze its effects on shortening and calcium transport in ventricular myocytes from the Goto-Kakizaki (GK) type 2 diabetic rat
- to treat HepG2 cells with peroxisome proliferator-activated receptor γ (PPARγ) agonists to examine its effect on TOMM40-, APOE- and APOC1-mRNA levels
생화학적/생리학적 작용
Pioglitazone hydrochloride is a PPARγ agonist and thiazolidinedione (TZD) anti-diabetic.
Pioglitazone hydrochloride is a PPARγ agonist and thiazolidinedione (TZD) anti-diabetic. Pioglitazone is a selective agonist of the nuclear receptor peroxisome proliferator-activated receptor γ (PPAR-γ) and to a lesser extent PPAR-α.
Pioglitazone hydrochloride is usually used to treat type-II diabetes. It has the ability to block hepatic gluconeogenesis.
특징 및 장점
This compound is a featured product for ADME Tox research. Click here to discover more featured ADME Tox products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the AMPKs and Nuclear Receptors (PPARs) pages of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by Takeda. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Romina Lomonaco et al.
Drugs, 73(1), 1-14 (2013-01-19)
Nonalcoholic fatty liver disease (NAFLD) is considered the most common liver disorder in the Western world. It is commonly associated with insulin resistance, obesity, dyslipidaemia, type 2 diabetes mellitus (T2DM) and cardiovascular disease. Nonalcoholic steatohepatitis (NASH) is characterized by steatosis
Andrew Grey et al.
European journal of endocrinology, 170(2), 255-262 (2013-11-13)
Preclinical studies, observational studies, and clinical trials suggest that thiazolidinediones (TZDs) reduce bone mineral density (BMD) and increase fracture risk. Most of the evidence on the skeletal effects of TZDs is from studies of rosiglitazone. We set out to investigate
Peter Ochodnicky et al.
European journal of pharmacology, 730, 51-60 (2014-03-04)
Peroxisome proliferator-activated receptor γ (PPARγ) agonists have been shown to ameliorate diabetic nephropathy, but much less are known about their effects in non-diabetic nephropathies. In the present study, metabolic parameters, blood pressure, aortic endothelial function along with molecular and structural
J R Colca et al.
Clinical pharmacology and therapeutics, 93(4), 352-359 (2013-03-07)
It may be possible to achieve insulin sensitivity through the recently identified mitochondrial target of thiazolidinediones (mTOT), thereby avoiding peroxisome proliferator-activated receptor-γ (PPAR-γ)-dependent side effects. In this phase IIb clinical trial, 258 patients with type 2 diabetes completed a 12-week
Julien Lamontagne et al.
Diabetes, 62(6), 2122-2129 (2013-02-05)
Our objective was to determine if the insulin-sensitizing drug pioglitazone acutely reduces insulin secretion and causes metabolic deceleration in vivo independently of change in insulin sensitivity. We assessed glucose homeostasis by hyperinsulinemic-euglycemic and hyperglycemic clamp studies and energy expenditure by
관련 콘텐츠
Discover Bioactive Small Molecules for ADME/Tox
Discover Bioactive Small Molecules for Lipid Signaling Research
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.