모든 사진(3)
About This Item
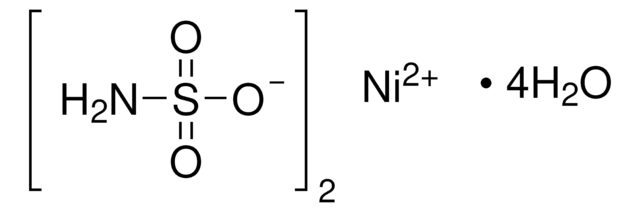
Linear Formula:
NiSO4 · 6H2O
CAS Number:
Molecular Weight:
262.85
EC Number:
MDL number:
UNSPSC 코드:
12352302
PubChem Substance ID:
NACRES:
NA.21
분석:
98.0-102.0% (EDTA titration)
양식:
powder or crystals
추천 제품
vapor density
2.07 (vs air)
Quality Level
제품 라인
ReagentPlus®
분석
98.0-102.0% (EDTA titration)
양식
powder or crystals
양이온 미량물
Fe: ≤0.001%
SMILES string
O.O.O.O.O.O.[Ni++].[O-]S([O-])(=O)=O
InChI
1S/Ni.H2O4S.6H2O/c;1-5(2,3)4;;;;;;/h;(H2,1,2,3,4);6*1H2/q+2;;;;;;;/p-2
InChI key
RRIWRJBSCGCBID-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Nickel(II) sulfate hexahydrate (NiSO4 · 6H2O) is commonly used as a nickel precursor and catalyst in the various organic synthesis.
애플리케이션
Nickel(II) sulfate hexahydrate can be used:
- As a catalyst for the synthesis of α-aminophosphonates by a condensation reaction of aromatic aldehydes, primary amines, and diethylphosphite.
- In the synthesis of nickel (II) hexamethylenetetramine complex [Ni(HMTA)2 (NCS)2(H2O)2]H2O with thiocyanate coligand.
- As a nickel source in the synthesis of nanosized nickel phosphides by the solvothermal route using red phosphorus as the P-3 precursor.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A Inhalation - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 1 Inhalation
표적 기관
Respiratory Tract
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Eun A Choi et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 39(25), 4945-4958 (2019-04-14)
Decision-making often involves motivational conflict because of the competing demands of approach and avoidance for a common resource: behavior. This conflict must be resolved as a necessary precursor for adaptive behavior. Here we show a role for the paraventricular thalamus
Asymmetric induction by retgersite, nickel sulfate hexahydrate, in conjunction with asymmetric autocatalysis.
Matsumoto A, et al.
New. J. Chem., 39(9), 6742-6745 (2015)
Structure of monoclinic nickel (II) sulfate hexahydrate.
Gerkin RE and Reppart WJ.
Acta Crystallographica Section C, Crystal Structure Communications, 44(8), 1486-1488 (1988)
Mechanism and kinetics of thermal decomposition of nickel (II) sulfate (VI) hexahydrate.
Tomaszewicz E and Kotfica M.
Journal of Thermal Analysis and Calorimetry, 77(1), 25-31 (2004)
Polymorphism of nickel sulfate hexahydrate.
Angel RJ and Finger LW.
Acta Crystallographica Section C, Crystal Structure Communications, 44(11), 1869-1873 (1988)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.