모든 사진(4)
About This Item
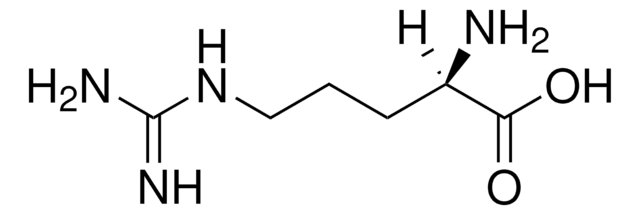
Linear Formula:
H2NC(=NH)NH(CH2)3CH(NH2)CO2H
CAS Number:
Molecular Weight:
174.20
Beilstein:
1725413
EC Number:
MDL number:
UNSPSC 코드:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
추천 제품
product name
L-Arginine, reagent grade, ≥98%
Grade
reagent grade
Quality Level
분석
≥98%
형태
powder
색상
white
mp
222 °C (dec.) (lit.)
solubility
H2O: 50 mg/mL
응용 분야
cell analysis
peptide synthesis
SMILES string
N[C@@H](CCCNC(N)=N)C(O)=O
InChI
1S/C6H14N4O2/c7-4(5(11)12)2-1-3-10-6(8)9/h4H,1-3,7H2,(H,11,12)(H4,8,9,10)/t4-/m0/s1
InChI key
ODKSFYDXXFIFQN-BYPYZUCNSA-N
유전자 정보
human ... NOS1(4842) , NOS2(4843)
rat ... Ppm1a(24666)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
L-arginine has been used to study non-enzymatic gluconeogenesis. It has also been used to study the effects of L-arginine supplementation on kidney and liver injury in rats with myocardial infarction.
생화학적/생리학적 작용
L-Arginine is a dibasic, semi-essential amino acid. It acts as a precursor for creatinine and is a natural constituent of most of the dietary proteins.
Substrate of nitric oxide synthase, which is converted to citrulline and nitric oxide (NO). Induces insulin release by a nitric oxide-dependent mechanism.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
J.P.F. D'Mello
Amino Acids in Human Nutrition and Health (2012)
Nonenzymatic gluconeogenesis-like formation of
fructose 1,6-bisphosphate in ice
fructose 1,6-bisphosphate in ice
Christoph B. Messner
Proceedings of the National Academy of Sciences of the USA, 7403-7407 (2017)
Aerobic training and L-arginine supplement attenuates myocardial infarction-induced kidney and liver injury in rats via reduces oxidative stress
Kamal Ranjbar
Indian Heart Journal (2017)
Palaniraja Thandapani et al.
Molecular cell, 50(5), 613-623 (2013-06-12)
Motifs rich in arginines and glycines were recognized several decades ago to play functional roles and were termed glycine-arginine-rich (GAR) domains and/or RGG boxes. We review here the evolving functions of the RGG box along with several sequence variations that
Krishnan Suresh Kumar et al.
European journal of medicinal chemistry, 45(11), 5474-5479 (2010-08-21)
A new series of 3-(benzylideneamino)-2-phenylquinazoline-4(3H)-ones were prepared through Schiff base formation of 3-amino-2-phenyl quinazoline-4(3)H-one with various substituted carbonyl compounds. Their chemical structures were elucidated by spectral studies. Cytotoxicity and antiviral activity were evaluated against herpes simplex virus-1 (KOS), herpes simplex
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.