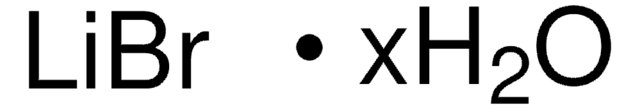추천 제품
vapor pressure
1 hPa ( 748 °C)
제품 라인
ReagentPlus®
분석
≥99%
형태
powder
pH
7 (20 °C, 10 g/L)
mp
550 °C (lit.)
SMILES string
[Li+].[Br-]
InChI
1S/BrH.Li/h1H;/q;+1/p-1
InChI key
AMXOYNBUYSYVKV-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
LiBr/Chlorotrimethylsilane reagent participates in the conversion of alcohols to bromides.
애플리케이션
Lithium bromide (LiBr) may be employed as a catalyst in the following studies:
- Transformation of (aromatic- and α,β-unsaturated) aldehydes to dithioacetals via solvent-free dithioacetalization.
- Synthesis of olefins via condensation reaction of carbonyl compounds with active methylene compounds.
- Green synthesis of β-amino alcohols.
- Chemo- and regioselective bromination of aromatic compounds was carried out by employing LiBr/ceric ammonium nitrate (CAN) reagent system (as a source of Br+ ion).
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Redi-Dri is a trademark of Sigma-Aldrich Co. LLC
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Lithium Bromide, an Inexpensive and Efficient Catalyst for Opening of Epoxide Rings by Amines at Room Temperature under Solvent-Free Condition.
Chakraborti AK, et al.
European Journal of Organic Chemistry, 2004(17), 3597-3600 (2004)
An efficient chemo and regioselective oxidative nuclear bromination of activated aromatic compounds using lithium bromide and ceric ammonium nitrate.
Roy SC, et al.
Tetrahedron Letters, 42(39), 6941-6942 (2001)
Lithium bromide-catalyzed highly chemoselective and efficient dithioacetalization of α,β-unsaturated and aromatic aldehydes under solvent-free conditions.
Firouzabadi H, et al.
Synthesis, 58-60 (1999)
Lithium bromide as a new catalyst for carbon-carbon bond formation in the solid state.
Prajapati D, et al.
Journal of the Chemical Society. Perkin Transactions 1, 9, 959-960 (1996)
R Quaderer et al.
Organic letters, 3(20), 3181-3184 (2001-09-28)
[reaction: see text] The alkanesulfonamide "safety-catch" resin has proven useful for Fmoc-based synthesis of C-terminal peptide thioesters. We now report that the yield of isolated thioester can increase significantly when the cleavage reaction is carried out in 2 M LiBr/THF
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.