추천 제품
Grade
pharmaceutical primary standard
API family
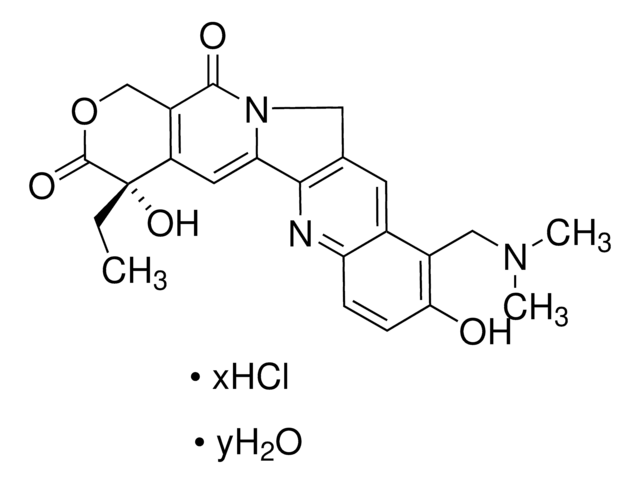
capecitabine
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
O[C@H]1[C@@H](O)[C@H](N2C(N=C(NC(OCCCCC)=O)C(F)=C2)=O)O[C@@H]1C
InChI
1S/C15H22FN3O6/c1-3-4-5-6-24-15(23)18-12-9(16)7-19(14(22)17-12)13-11(21)10(20)8(2)25-13/h7-8,10-11,13,20-21H,3-6H2,1-2H3,(H,17,18,22,23)/t8-,10-,11-,13-/m1/s1
InChI key
GAGWJHPBXLXJQN-UORFTKCHSA-N
유전자 정보
human ... TYMS(7298)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Capecitabine EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
생화학적/생리학적 작용
Capecitabine is an anti-cancer drug, a prodrug of doxifluridine, metabolized to 5-fluorouracil at the tumor site. The activation of capecitabine follows a pathway with three enzymatic steps and two intermediary metabolites, 5′-Deoxy-5-fluorocytidine (5′-DFCR) and 5′-Deoxy-5-fluorouridine (5′-DFUR), to form 5-fluorouracil.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Carc. 1B - Muta. 2 - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Francesco Giotta et al.
Tumori, 99(6), 278e-281e (2014-02-08)
We present the case of a 58-year-old woman with breast cancer metastasizing to the liver after adjuvant chemotherapy. A liver biopsy confirmed metastatic lesions from breast cancer that were immunohistochemically positive for estrogen/progesterone receptors and HER2. After first-line treatment with
Karen-Lise G Spindler et al.
Anticancer research, 34(2), 845-850 (2014-02-11)
We investigated the efficacy and safety of capecitabine and gemcitabin (GemCap) in heavily pre-treated, therapy-resistant metastatic colorectal cancer (mCRC) patients and the clinical importance of cell-free DNA (cfDNA) measurement. Patients' inclusion criteria included histopathologically-verified mCRC refractory to standard chemotherapy, adequate
Dermatomyositis associated with capecitabine in the setting of malignancy.
Frank W Chen et al.
Journal of the American Academy of Dermatology, 70(2), e47-e48 (2014-01-21)
Mitsuhiro Tomoda et al.
Anticancer research, 34(1), 191-194 (2014-01-10)
Unresectable metastatic colorectal cancer with very slow tumour growth rate does not necessarily require for strong short-interval chemotherapy. In the present study, we administered monthly chemotherapy and aimed to evaluate the usefulness of the specific treatment schedule in patients with
Capecitabine and streptozocin ± cisplatin in advanced gastroenteropancreatic neuroendocrine tumours.
Tim Meyer et al.
European journal of cancer (Oxford, England : 1990), 50(5), 902-911 (2014-01-22)
Cytotoxic chemotherapy is widely used for advanced, unresectable pancreatic and other gastrointestinal foregut neuroendocrine tumours (NETs) and the most commonly used regimen combines 5-fluorouracil with streptozocin. The NET01 trial was designed to investigate whether capecitabine combined with streptozocin was an
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.