추천 제품
Grade
pharmaceutical primary standard
API family
tiaprofenic acid
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
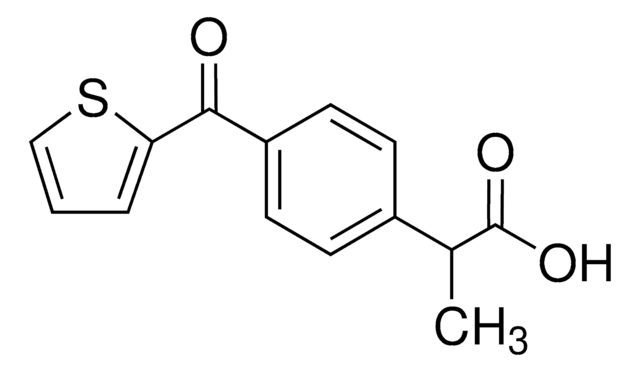
SMILES string
[s]1c(ccc1C(=O)c2ccccc2)C(C)C(=O)O
InChI
1S/C14H12O3S/c1-9(14(16)17)11-7-8-12(18-11)13(15)10-5-3-2-4-6-10/h2-9H,1H3,(H,16,17)
InChI key
GUHPRPJDBZHYCJ-UHFFFAOYSA-N
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Tiaprofenic acid EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 3 Oral - Repr. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
B E Gencosmanoglu et al.
Research in experimental medicine. Zeitschrift fur die gesamte experimentelle Medizin einschliesslich experimenteller Chirurgie, 200(3), 215-226 (2001-06-28)
We evaluate the chondrotoxic effects of some nonsteroidal anti-inflammatory drugs (NSAIDs) on articular cartilage in an experimental model of osteoarthritis (OA). Each of 40 Sprague-Dawley rats weighing 0.250 kg and 12 weeks old received weekly injections of sodium iodoacetate (1
[Cross-reaction potentials of ketoprofen].
Rudolf Szebeni
Orvosi hetilap, 143(19), 1043-1043 (2002-06-18)
H Uzun et al.
Acta orthopaedica Scandinavica, 72(5), 499-502 (2001-12-01)
39 patients with active knee osteoarthrosis, chosen according to ACR criteria, were assigned to receive flurbiprofen (n 12, 2 x 100 mg), tiaprofenic acid (n 14, 2 x 300 mg) and placebo (n 13) in a 3-week, placebo-controlled study. All
Y Ozsoy et al.
Farmaco (Societa chimica italiana : 1989), 59(7), 563-566 (2004-07-03)
The aim of the present study was to investigate the in vitro release properties of tiaprofenic acid (TA) from different topical vehicles. Carbopol 940 gel, chitosan gel, two types of emulsion-based ointment formulations (o/w and w/o) and hydrophilic petrolatum USP
U Tursen et al.
The Journal of dermatological treatment, 13(4), 205-206 (2002-12-01)
Tiaprofenic acid is a propionic acid derivative with analgesic, anti-inflammatory and antipyretic properties. Fixed drug eruption is a common cutaneous reaction by various drugs. Fixed drug eruption induced by tiaprofenic acid has not been reported previously. Described in this paper
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.