S9876
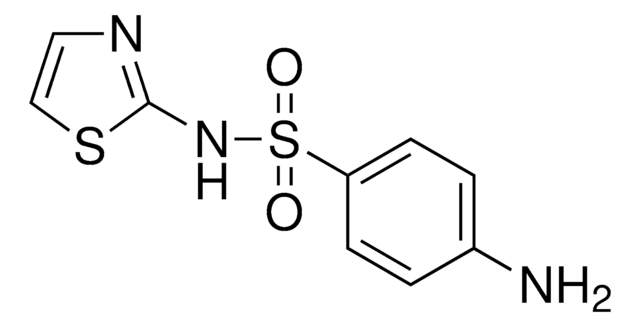
Sulfathiazole
동의어(들):
2-Sulfanilamidothiazole, 4-Amino-N-(2-thiazolyl)benzenesulfonamide, N1-(2-Thiazolyl)sulfanilamide
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C9H9N3O2S2
CAS Number:
Molecular Weight:
255.32
Beilstein:
226178
EC Number:
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
analytical standard
Quality Level
Agency
EPA 1694
형태
powder
기술
HPLC: suitable
gas chromatography (GC): suitable
mp
200-202 °C (lit.)
응용 분야
clinical testing
SMILES string
Nc1ccc(cc1)S(=O)(=O)Nc2nccs2
InChI
1S/C9H9N3O2S2/c10-7-1-3-8(4-2-7)16(13,14)12-9-11-5-6-15-9/h1-6H,10H2,(H,11,12)
InChI key
JNMRHUJNCSQMMB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Sulfathiazole is a broad-spectrum antibacterial drug, belonging to the class of sulfonamides, used in veterinary and human medication for the treatment of infections. It is also involved in promoting growth of livestock and fish.
애플리케이션
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Sulfathiazole is used as an analytical reference standard for the quantification of the analyte in meat, and honey samples using different chromatography techniques.
생화학적/생리학적 작용
Sulfonamide antibiotic that blocks the synthesis of dihydrofolic acid by inhibiting the enzyme dihydropteroate synthase.
Mode of Action: Inhibits folic acid synthesis in prokaryotes.
Anti-microbial Spectrum: Gram positive, Gram negative, Chlamydia
Mode of Resistance: Alteration of dihydropteroate synthase or alternative pathway for folic acid synthesis.
Mode of Action: Inhibits folic acid synthesis in prokaryotes.
Anti-microbial Spectrum: Gram positive, Gram negative, Chlamydia
Mode of Resistance: Alteration of dihydropteroate synthase or alternative pathway for folic acid synthesis.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Quantitative LC/MS-MS determination of sulfonamides and some other antibiotics in honey
Kaufmann A, et al.
Journal of AOAC (Association of Official Analytical Chemists) International, 85(4), 853-860 (2002)
Comparison of separation conditions and ionization methods for the liquid chromatography?mass spectrometric determination of sulfonamides
Kim H-D and Lee WD
Journal of Chromatography A, 984(1), 153-158 (2003)
Determination of sulfonamides in meat by liquid chromatography coupled with atmospheric pressure chemical ionization mass spectrometry
Kim H-D, et al.
Bull. Korean Chem. Soc., 23(11), 1590-1594 (2002)
Merle K Richter et al.
Environmental science & technology, 43(17), 6632-6638 (2009-09-22)
Sorption of sulfathiazole (STA) and three structural analogs to Leonardite humic acid (LHA) was investigated in single- and binary-solute systems to elucidate the sorption mechanism of sulfonamides to soil organic matter (SOM). Cation binding of STA+ to anionic sites A-
Hongyun Niu et al.
Journal of hazardous materials, 190(1-3), 559-565 (2011-04-26)
Humic acid coated Fe(3)O(4) magnetic nanoparticles (Fe(3)O(4)/HA) were prepared for the removal of sulfathiazole from aqueous media. Fe(3)O(4)/HA exhibited high activity to produce hydroxyl (OH) radicals through catalytic decomposition of H(2)O(2). The degradation of sulfathiazole was strongly temperature-dependent and favored
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.