추천 제품
Grade
pharmaceutical primary standard
API family
rifamycin, rifampicin
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
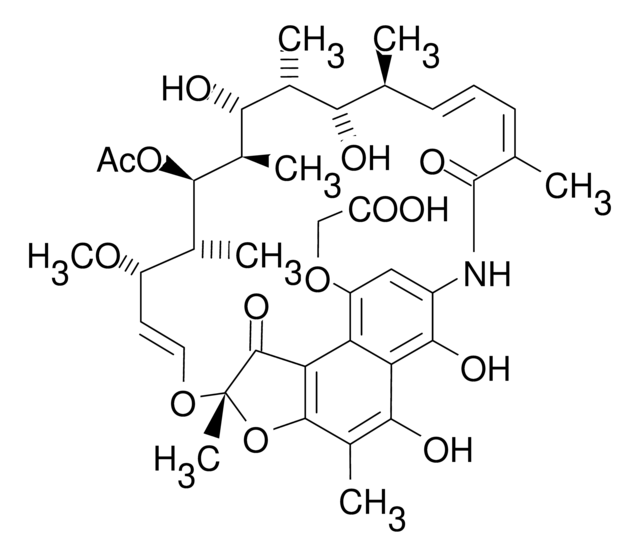
SMILES string
N1C2=CC(=O)c3c4c(c(c(c3C2=O)O)C)O[C@](O\C=C\[C@@H]([C@H]([C@H]([C@@H]([C@@H]([C@@H]([C@H]([C@H](\C=C\C=C(/C1=O)\C)C)O)C)O)C)OC(=O)C)C)OC)(C4=O)C
InChI
1S/C37H45NO12/c1-16-11-10-12-17(2)36(46)38-23-15-24(40)26-27(32(23)44)31(43)21(6)34-28(26)35(45)37(8,50-34)48-14-13-25(47-9)18(3)33(49-22(7)39)20(5)30(42)19(4)29(16)41/h10-16,18-20,25,29-30,33,41-43H,1-9H3,(H,38,46)/b11-10+,14-13+,17-12-/t16-,18+,19+,20+,25-,29-,30+,33+,37-/m0/s1
InChI key
BTVYFIMKUHNOBZ-ODRIEIDWSA-N
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Rifamycin S EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - STOT SE 2
표적 기관
Liver
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
가장 최신 버전 중 하나를 선택하세요:
Ming Chen et al.
The Journal of organic chemistry, 78(1), 3-8 (2012-06-19)
Syntheses of the C(15)-C(27) fragments of chaxamycins A/D, rifamycin S, and the C(12)-C(24) fragment of salinisporamycin have been accomplished in 10 steps from commercially available starting materials. Three crotylboron reagents were utilized to construct the seven contiguous stereocenters in these
U C Banerjee
Biomaterials, artificial cells, and immobilization biotechnology : official journal of the International Society for Artificial Cells and Immobilization Biotechnology, 21(5), 675-683 (1993-01-01)
Rifamycin oxidase of Curvularia lunata was immobilized on alginate gel. The pH and temperature optima of the immobilized enzyme preparation were 6.5 and 50 degrees C, respectively. Transformation reaction was carried out with the immobilized enzyme preparation. It took 8
Molecular structure and conformation of rifamycin S, a potent inhibitor of DNA-dependent RNA polymerase.
S K Arora et al.
The Journal of antibiotics, 45(3), 428-431 (1992-03-01)
S BouzBouz et al.
Organic letters, 3(25), 3995-3998 (2001-12-12)
[reaction: see text] An efficient, simple method has been developed for the stereocontrolled synthesis of polypropionate stereopentads in high enantio- and diastereomeric purities.
O Ghisalba et al.
The Journal of antibiotics, 35(1), 74-80 (1982-01-01)
The transformation of rifamycin S into rifamycins B and L was reinvestigated in order to establish more detailed pathways. Our results exclude rifamycin O as a common progenitor in the biosyntheses of rifamycins B and L. Rifamycins B and L
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.