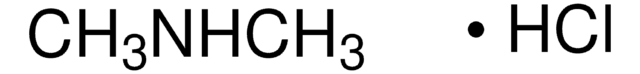추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to USP 1466674
vapor pressure
5 mmHg ( 20 °C)
양식
liquid
CofA
current certificate can be downloaded
포장
pkg of 100 mg
refractive index
n20/D 1.437 (lit.)
bp
153 °C/774 mmHg (lit.)
density
1.01 g/mL (lit.)
응용 분야
pharmaceutical
저장 온도
2-8°C
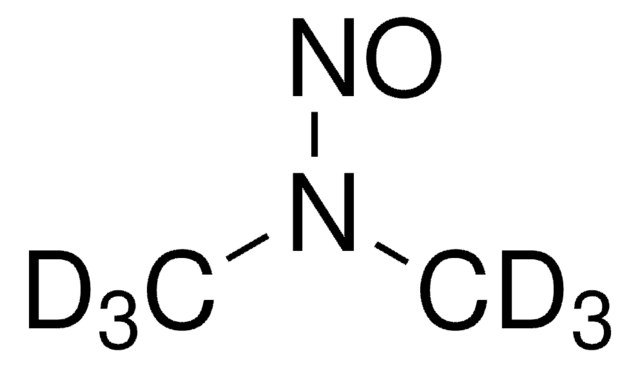
SMILES string
CN(C)N=O
InChI
1S/C2H6N2O/c1-4(2)3-5/h1-2H3
InChI key
UMFJAHHVKNCGLG-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. It is analyzed using GMP validated instruments as per pharmacopeia monograph methods and is traceable to Unites States Pharmacopeia (USP), European Pharmacopeia (EP), and British Pharmacopeia (BP) primary standards, wherever applicable.
It is provided with a comprehensive certificate of analysis (CoA) containing a certified purity value, calculated by the mass balance approach. All information regarding the use of this CRM can be found on the certificate of analysis.N-Nitrosodimethylamine (NDMA) is a nitrosamine that occurs as an impurity in sartan angiotensin II receptor blocker drugs.
It is provided with a comprehensive certificate of analysis (CoA) containing a certified purity value, calculated by the mass balance approach. All information regarding the use of this CRM can be found on the certificate of analysis.N-Nitrosodimethylamine (NDMA) is a nitrosamine that occurs as an impurity in sartan angiotensin II receptor blocker drugs.
애플리케이션
N-Nitrosodimethylamine CRM may also find uses as given below:
- Determination of N-Nitrosodimethylamine (NDMA) as an impurity in four valsartan APIs and tablets by high-performance liquid chromatography (HPLC)
- Quantitative analysis of NDMA in valsartan pharmaceutical formulations by capillary electrophoresis-nanospray-mass spectrometry
- Simultaneous determination of N-nitrosodimethylamine and N-nitrosomethylethylamine in drug substances and products containing sartans, ranitidine, and metformin by solid-phase extraction (SPE) and gas chromatography-tandem mass spectrometry (GC-MS/MS)
- Analysis of NDMA in the olmesartan API and tablets by high-performance liquid chromatography-mass spectrometry (HPLC-MS)
- Development and validation of an HPLC-MS/MS method for separation and quantification of NDMA impurity for quality control of ranitidine products
생화학적/생리학적 작용
Induces gastric, liver, kidney and lung cancer in mice and rats.
기타 정보
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
각주
To see an example of a Certificate of Analysis for this material enter LRAC4355 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 2 Oral - Aquatic Chronic 2 - Carc. 1B - STOT RE 1
표적 기관
Liver
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
141.8 °F - closed cup
Flash Point (°C)
61.0 °C - closed cup
가장 최신 버전 중 하나를 선택하세요:
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.