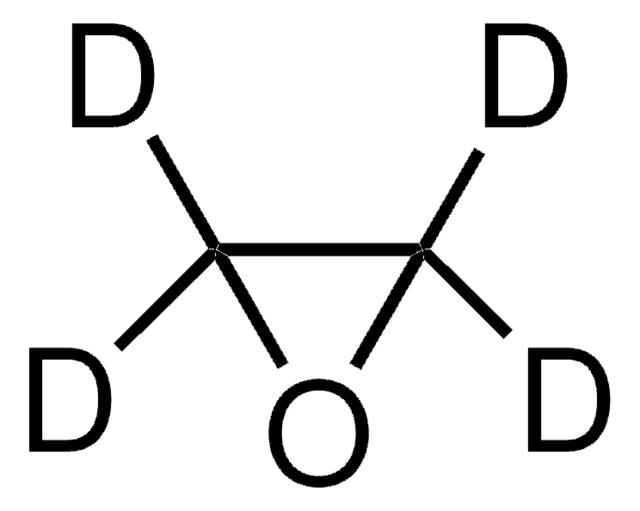
추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
양식
solution (, solvent is PEG200)
CofA
current certificate can be downloaded
응용 분야
pharmaceutical (small molecule)
저장 온도
2-8°C
일반 설명
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
애플리케이션
Ethylene Oxide may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by gas-liquid chromatography technique.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
분석 메모
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
기타 정보
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
각주
To see an example of a Certificate of Analysis for this material enter LRAB7763 in the slot below. This is an example certificate only and may not be the lot that you receive.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Carc. 1B - Muta. 1B - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Simple GLC determination of ethylene oxide and its reaction products in drugs and formulations.
Hartman PA and Bowman PB
Journal of Pharmaceutical Sciences, 66(6), 789-792 (1977)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.