PHR1149
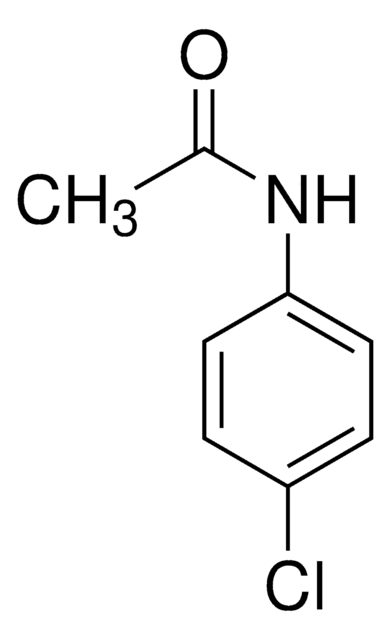
Acetaminophen Related Compound J
Pharmaceutical Secondary Standard; Certified Reference Material
동의어(들):
4′-Chloroacetanilide, N-(4-Chlorophenyl)acetamide, Acetic acid 4-chloroanilide, NSC 40563, NSC 444
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
CH3CONHC6H4Cl
CAS Number:
Molecular Weight:
169.61
Beilstein:
509638
EC Number:
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to Ph. Eur. Y0001945
traceable to USP 1003100
API family
paracetamol, acetaminophen
CofA
current certificate can be downloaded
기술
HPLC: suitable
gas chromatography (GC): suitable
mp
176-178 °C (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-30°C
SMILES string
CC(=O)Nc1ccc(Cl)cc1
InChI
1S/C8H8ClNO/c1-6(11)10-8-4-2-7(9)3-5-8/h2-5H,1H3,(H,10,11)
InChI key
GGUOCFNAWIODMF-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is an impurity of the widely used antipyretic and analgesic drug acetaminophen, also known as paracetamol.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is an impurity of the widely used antipyretic and analgesic drug acetaminophen, also known as paracetamol.
애플리케이션
This pharmaceutical secondary standard can also be used as follows:
- Separation and estimation of acetaminophen and its process impurities in commercial acetaminophen tablets using high-performance liquid chromatography (HPLC)
- Development and validation of HPLC-based stability indicating method for the determination of acetaminophen, chlorpheniramine maleate, and their possible degradation products in an over-the-counter syrup formulation
- Detection and quantification of paracetamol, phenylephrine hydrochloride, and paracetamol impurities in pharmaceutical formulations using four chemometric-based spectrophotometric methods
- Impurity analysis of a combined suppository dosage form of paracetamol, codeine phosphate hemihydrate, and pitophenone hydrochloride using ion-pair reversed-phase liquid chromatography in combination with UV detection
- Development of a thin-layer chromatography method combined with densitometry for the separation of acetaminophen and its related impurities from commercial dosage forms
분석 메모
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
기타 정보
Values of analytes vary lot to lot.
각주
To see an example of a Certificate of Analysis for this material enter LRAC0397 in the slot below. This is an example certificate only and may not be the lot that you receive.
추천 제품
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Simultaneous Estimation of Acetaminophen, Chlorpheniramine Maleate, Methyl Paraben, Propyl Paraben, Sodium Benzoate and Related Impurities in Over-the-Counter Syrup Formulation
Palakurthi AK and Dongala T
Pharmaceutical Chemistry Journal, 1-7 (2022)
Alina Pyka et al.
BioMed research international, 2013, 545703-545703 (2013-09-26)
Adsorption thin layer chromatography (NP-TLC) with densitometry has been established for the identification and the quantification of acetaminophen in three leading commercial products of pharmaceutical tablets coded as brand: P1 (Product no. 1), P2 (Product no. 2), and P3 (Product
Tamas A Godany et al.
Chimia, 65(4), 253-255 (2011-06-18)
Lithiation of N-(4-chlorophenyl)-pivalamide (NCP) and two additional substituted acetanilides: 4-fluoroacetanilide (4-F) and 4-chloroacetanilide (4-Cl) has been monitored by means of calorimetry, on-line ATR-IR and UV/vis spectroscopy and endoscopy. The combined on-line monitoring revealed the differences between the reaction paths of
L Koymans et al.
Xenobiotica; the fate of foreign compounds in biological systems, 23(6), 633-648 (1993-06-01)
1. The general mechanism of metabolic oxidation of substrates by cytochromes P450 (P450s) appears to consist of sequential one-electron oxidation steps rather than of a single concerted transfer of activated oxygen species from P450 to substrates. 2. In case of
Octavian Călinescu et al.
Journal of chromatographic science, 50(4), 335-342 (2012-03-13)
Determination of acetaminophen and its main impurities: 4-nitrophenol, 4'-chloroacetanilide, as well as 4-aminophenol and its degradation products, p-benzoquinone and hydroquinone has been developed and validated by a new high-performance liquid chromatography method. Chromatographic separation has been obtained on a Hypersil
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.