PHR1073
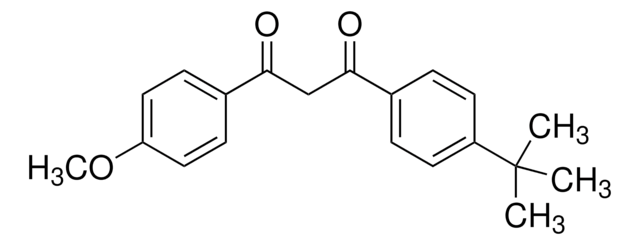
Avobenzone
Pharmaceutical Secondary Standard; Certified Reference Material
동의어(들):
1-(4-Methoxyphenyl)-3-(4-tert-butylphenyl)-1,3-propanedione, Butyl methoxydibenzoylmethane
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C20H22O3
CAS Number:
Molecular Weight:
310.39
EC Number:
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to USP 1045337
API family
avobenzone
CofA
current certificate can be downloaded
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
environmental
pharmaceutical (small molecule)
형식
neat
저장 온도
2-30°C
SMILES string
COc1ccc(cc1)C(=O)CC(=O)c2ccc(cc2)C(C)(C)C
InChI
1S/C20H22O3/c1-20(2,3)16-9-5-14(6-10-16)18(21)13-19(22)15-7-11-17(23-4)12-8-15/h5-12H,13H2,1-4H3
InChI key
XNEFYCZVKIDDMS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Avobenzone is an active sunscreen ingredient, used in sunscreen lotions and cosmetic formulations.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
애플리케이션
Avobenzone may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations using reversed-phase high-performance liquid chromatography technique.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
분석 메모
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
기타 정보
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
각주
To see an example of a Certificate of Analysis for this material enter LRAB3693 in the slot below. This is an example certificate only and may not be the lot that you receive.
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 4
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
이미 열람한 고객
Development and validation of a new RP-HPLC method for the simultaneous determination of hydroquinone, kojic acid, octinoxate, avobenzone, BHA and BHT in skin-whitening cream.
Galimany-Rovira F, et al.
Analytical Methods : Advancing Methods and Applications, 8(5), 1170-1180 (2016)
Serge Forestier
Journal of the American Academy of Dermatology, 58(5 Suppl 2), S133-S138 (2008-04-25)
The risks associated with cumulative or overexposure to ultraviolet (UV) radiation are now well identified. They involve both UVB (290-320 nm) and UVA (320-400 nm) components. UVB radiation is still considered to be the major factor responsible for most harmful
Ibrahim Hanno et al.
Pharmaceutical research, 29(2), 559-573 (2011-09-23)
To prepare polyamide nanocapsules for skin photo-protection, encapsulating α-tocopherol, Parsol®MCX (ethylhexyl methoxycinnamate) and/or Parsol®1789 (butyl methoxydibenzoylmethane). Nanocapsules were obtained by combining spontaneous emulsification and interfacial polycondensation reaction between sebacoyl chloride and diethylenetriamine. Nano-emulsions used as control were obtained by the
Esther J H Collaris et al.
International journal of dermatology, 47 Suppl 1, 35-37 (2008-11-15)
Over the last decade, a change in the public awareness regarding the possible danger of excessive sunlight exposure has resulted in an increased consumption of sunscreens. These products contain a broad spectrum of putative sensitizers that can cause contact dermatitis
Cecilia Paris et al.
Photochemistry and photobiology, 85(1), 178-184 (2008-08-05)
Novel sunscreens are required providing active protection in the UVA and UVB regions. On the other hand, there is an increasing concern about the photosafety of UV filters, as some of them are not sufficiently photostable. Avobenzone is one of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.