추천 제품
Grade
analytical standard
Quality Level
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
forensics and toxicology
pharmaceutical (small molecule)
veterinary
형식
neat
SMILES string
CC(C(O)=O)c1ccc(cc1)N2Cc3ccccc3C2=O
InChI
1S/C17H15NO3/c1-11(17(20)21)12-6-8-14(9-7-12)18-10-13-4-2-3-5-15(13)16(18)19/h2-9,11H,10H2,1H3,(H,20,21)
InChI key
RJMIEHBSYVWVIN-UHFFFAOYSA-N
유전자 정보
human ... IL8RA(3577) , PTGS1(5742) , PTGS2(5743)
애플리케이션
Indoprofen is a non-steroidal drug, which can show a range of pharmacological properties such as analgesic and anti-inflammatory activities. Its mode of action involves the inhibition of prostaglandin synthesis.
Indoprofen may be used as an analytical reference standard for the quantification of the analyte in biological samples and pharmaceutical formulations using different chromatography techniques.
Indoprofen may be used as an analytical reference standard for the quantification of the analyte in biological samples and pharmaceutical formulations using different chromatography techniques.
Indoprofen is a non-steroidal drug, which can show a range of pharmacological properties such as analgesic and anti-inflammatory activities.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 3 Oral - Carc. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
가장 최신 버전 중 하나를 선택하세요:
M Rosin et al.
Journal of clinical periodontology, 32(6), 617-621 (2005-05-11)
The pharmacodynamic properties of ibuprofen are related nearly exclusively to the S(+)enantiomer (dexibuprofen). This study investigated the effect of a 1.5% dexibuprofen mouth rinse in an experimentally induced gingivitis. The trial was a randomized, double-blinded, placebo-controlled, two-period and two-sequence parallel
Mitchell R Lunn et al.
Chemistry & biology, 11(11), 1489-1493 (2004-11-24)
Most patients with the pediatric neurodegenerative disease spinal muscular atrophy have a homozygous deletion of the survival motor neuron 1 (SMN1) gene, but retain one or more copies of the closely related SMN2 gene. The SMN2 gene encodes the same
D Siebler et al.
Arzneimittel-Forschung, 39(6), 659-660 (1989-06-01)
Binding of Racemic Indoprofen and its Enantiomers to Human Serum Albumin. The binding to 2% and 4% human serum albumin (HSA) of (+/-)-indoprofen, (-)-indoprofen, and (+)-indoprofen was examined applying a modified ultrafiltration process. The binding properties to HSA were characterized
B Lund et al.
British journal of clinical pharmacology, 22(6), 721-724 (1986-12-01)
Multiple dosing four times daily for 7 days of indoprofen 200 mg, a non-steroidal anti-inflammatory drug with a short half-life (t1/2), revealed drug accumulation in eight elderly subjects. This indicates a substantially longer t1/2 (11 h) than that reported in
Da-Qing Jin et al.
Pharmacology, biochemistry, and behavior, 89(3), 404-411 (2008-02-26)
Non-steroidal anti-inflammatory drugs (NSAIDs) have been proposed as a therapeutics to reduce the risk of Alzheimer's disease (AD). The present study shows that the peripheral administration of dexibuprofen (S(+)-isomer ibuprofen), which causes less gastric damage and has better anti-inflammatory effects
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.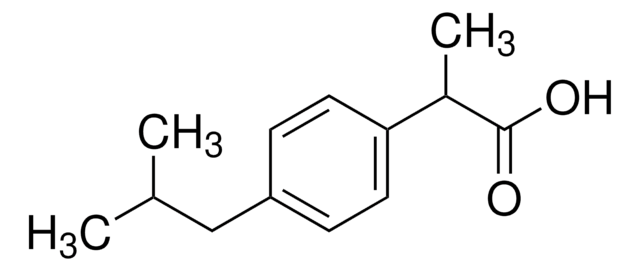


![[2-(Acryloyloxy)ethyl]trimethylammonium chloride solution 80 wt. % in H2O, contains 600 ppm monomethyl ether hydroquinone as inhibitor](/deepweb/assets/sigmaaldrich/product/structures/393/326/f7e19585-5431-4220-81b5-f458de6d63d0/640/f7e19585-5431-4220-81b5-f458de6d63d0.png)