About This Item
추천 제품
Grade
reagent
Quality Level
제품 라인
ReagentPlus®
분석
≥99%
양식
liquid
dilution
(for general lab use)
refractive index
n20/D 1.437 (lit.)
bp
130-131 °C (lit.)
mp
−51 °C (lit.)
density
0.951 g/mL at 25 °C (lit.)
작용기
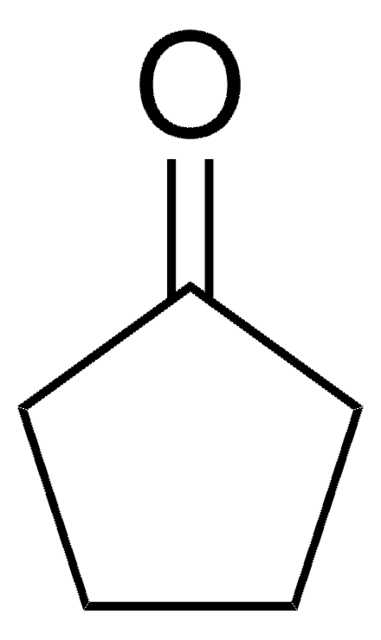
ketone
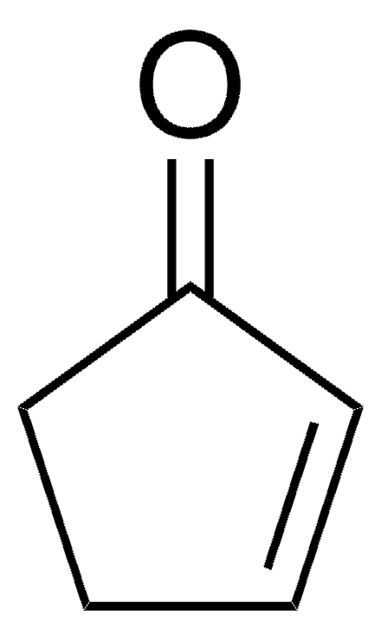
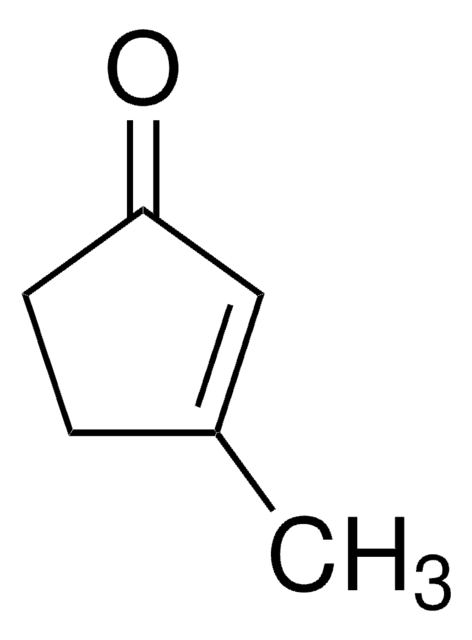
SMILES string
O=C1CCCC1
InChI
1S/C5H8O/c6-5-3-1-2-4-5/h1-4H2
InChI key
BGTOWKSIORTVQH-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
It may be used in the following studies:
- Preparation of (2E,5E)-2,5-bis(4-(azidomethyl)benzylidene) cyclopentanone, via cross-aldol condensation.
- Preparation of symmetrical C-5 curcuminoids by reacting with substituted benzaldehyde via Claisen-Schmidt condensation.
- As an electron pair donor to stabilize allyl and vinyl cations during intramolecular carbohydroxylation of alkynes.
법적 정보
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
86.0 °F - closed cup
Flash Point (°C)
30 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
문서
The aldol condensation reaction is an organic reaction introduced by Charles Wurtz, who first prepared the β-hydroxy aldehyde from acetaldehdye in 1872.
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.