모든 사진(1)
About This Item
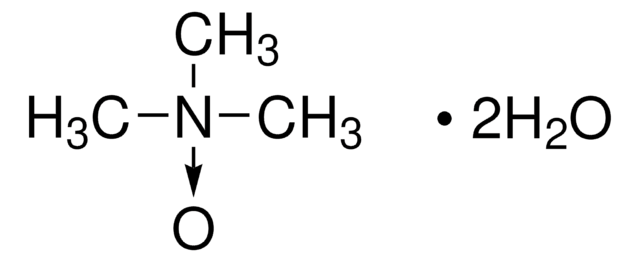
Linear Formula:
(CH3)3NO · 2H2O
CAS Number:
Molecular Weight:
111.14
Beilstein:
3612927
EC Number:
MDL number:
UNSPSC 코드:
12352116
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
Trimethylamine N-oxide (TMAO) is an amphiphilic osmolyte that can counteract the denaturing effects of urea, pressure, and ice and stabilize the proteins.
애플리케이션
Reactant for:
- C-H bond cleavage
- Oxidation reactions (oxidant)
- Decarbonylating agent for solvent-free reactions
Trimethylamine N-oxide dihydrate can react with transition-metal carbonyl cluster compounds and activate CO displacement by converting CO ligands into carbon dioxide.
기타 정보
Oxidant for the catalytic OsO4 cis-hydroxylation of hindered olefins; preparation of the unstable, crystalline anhydrous compound
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Cluster synthesis. 10. Reaction of Os3 (CO) 10 (. mu. 3-S) with trimethylamine N-oxide dihydrate. Syntheses and structural characterizations of Os3 (CO) 8 (NMe3)(. mu.-OH)(. mu. 3-S)(. mu.-H) and the six-atom-chain cluster Os6 (CO) 18 (. mu.-OH)(. mu. 4-S)(. mu. 3-S)(. mu.-H).
Adams RD, et al.
Inorganic Chemistry, 25(8), 1122-1127 (1986)
Counteraction of urea by trimethylamine N-oxide is due to direct interaction.
Meersman F, et al.
Biophysical Journal, 97(9), 2559-2566 (2009)
J.A. Soderquist et al.
Tetrahedron Letters, 27, 3961-3961 (1986)
Trimethylamine N-oxide promoted reactions of manganese, molybdenum and tungsten carbonyl complexes.
Blumer DJ, et al.
Journal of Organometallic Chemistry, 173(1), 71-76 (1979)
Complex formation in aqueous trimethylamine-N-oxide (TMAO) solutions.
Hunger J, et al.
The Journal of Physical Chemistry B, 116(16), 4783-4795 (2012)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.