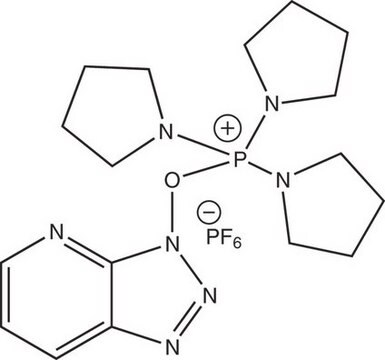
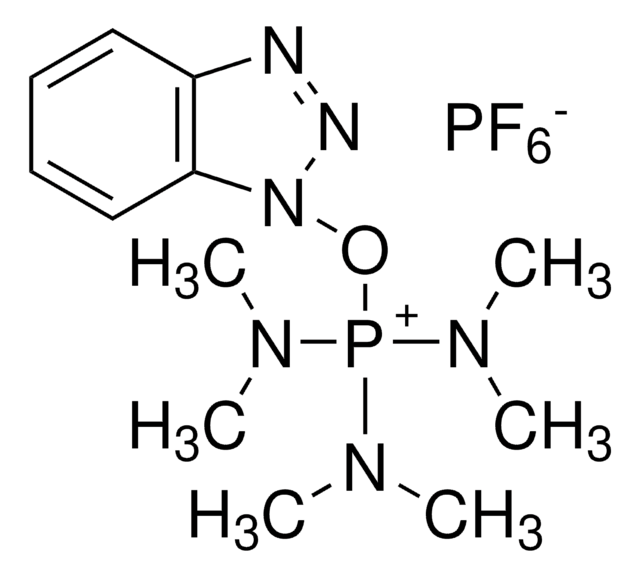
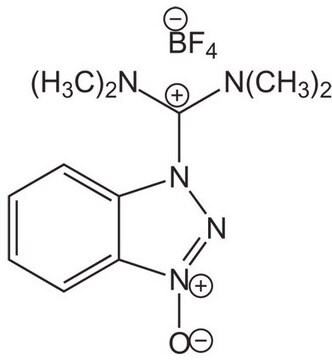
8.51004
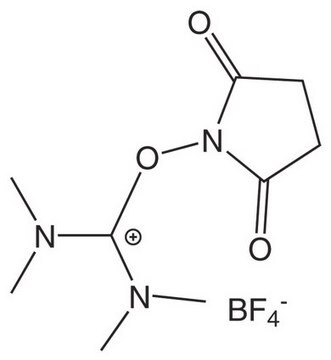
BOP
Benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphonium hexafluorophosphate Novabiochem®
동의어(들):
BOP, Benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphoniumhexafluorophosphate, Castro′s Reagent
About This Item
추천 제품
Quality Level
제품 라인
Novabiochem®
분석
≥99.0% (HPLC)
형태
powder
반응 적합성
reaction type: Coupling Reactions
제조업체/상표
Novabiochem®
mp
138-145 °C
응용 분야
peptide synthesis
저장 온도
2-8°C
InChI
1S/C12H22N6OP.F6P/c1-15(2)20(16(3)4,17(5)6)19-18-12-10-8-7-9-11(12)13-14-18;1-7(2,3,4,5)6/h7-10H,1-6H3;/q+1;-1
InChI key
MGEVGECQZUIPSV-UHFFFAOYSA-N
일반 설명
Associated Protocols and Technical Articles
Guide to Selection of Coupling Reagents Literature references
[1] B. Castro, et al. (1975) Tetrahedron Lett., 16, 1219.
[2] B. Castro, et al. (1976) Synthesis, 751.
[3] B. Castro, et al. (1977) Synthesis, 413.
[4] B. Castro, et al. (1977) J. Chem. Res. (S), 182.
[5] P. Rivaille, et al. (1980) Tetrahedron, 36, 3413.
[6] D. L. Nguyen, et al. (1985) J. Chem. Soc., Perkin Trans. 1, 1025.
[7] D. L. Nguyen, et al. (1987) J. Chem. Soc., Perkin Trans. 1, 1915.
[8] A. Fournier, et al. (1988) Int. J. Peptide Protein Res., 31, 86 and 231.
[9] J.-P. Briand, et al. (1989) Pept. Res., 2, 381.
[10] A. Fournier, et al. (1989) Int. J. Peptide Protein Res., 33, 133.
[11] D. L. Nguyen, et al. (1989) Biochem. Biophys. Res. Commun., 162, 1425.
[12] W. K. Rule, et al. in ′Peptides 1988, Proc. 20th European Peptide Symposium′, G. Jung & E. Bayer (Eds), Walter de Gruyter, Berlin, 1989, pp. 238.
[13] M. Forest, et al. (1990) Int. J. Peptide Protein Res., 35, 89.
[14] R. Seyer, et al. (1990) Int. J. Peptide Protein Res., 35, 465.
[15] H. Gausepohl, et al. in ′Innovation & Perspectives in Solid Phase Synthesis, 2nd International Symposium′, R. Epton (Eds), Intercept UK Ltd., Andover, 1993, pp. 387.
[16] R. P. McGeary (1998) Tetrahedron Lett., 39, 3319.
결합
분석 메모
Appearance of substance (visual): powder
Identity (IR): passes test
Assay (HPLC, area%): ≥ 99.0 % (a/a)
Solubility (0.6 mmole in 1 ml DMF): clearly soluble
법적 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
보충제 위험성
Storage Class Code
4.1A - Other explosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
문서
Novabiochem® offers a large number of coupling reagents for in situ activation. In situ activating reagents are easy to use, fast reacting – even with sterically hindered amino acids, and their use is generally free of side reactions.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.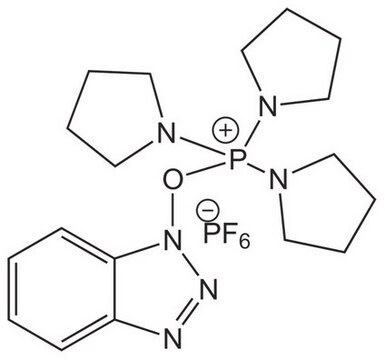
![COMU 1-[(1-(Cyano-2-ethoxy-2-oxoethylideneaminooxy) dimethylaminomorpholino)] uronium hexafluorophosphate Novabiochem®](/deepweb/assets/sigmaaldrich/product/images/237/337/13566c06-8931-4cc2-8621-c8742a392cd6/640/13566c06-8931-4cc2-8621-c8742a392cd6.jpg)