12804
HBTU
≥98.0% (T), for peptide synthesis
동의어(들):
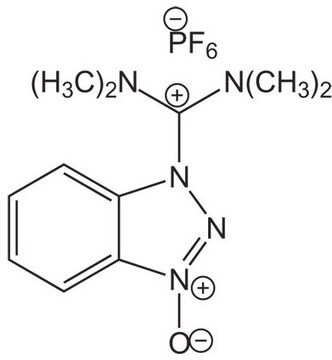
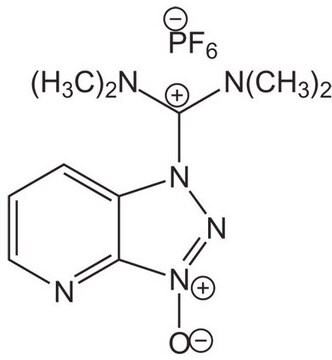
N,N,N′,N′-Tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate, O-(Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate
About This Item
추천 제품
product name
HBTU, ≥98.0% (T)
Quality Level
분석
≥98.0% (T)
형태
solid
반응 적합성
reaction type: Coupling Reactions
mp
200 °C (dec.) (lit.)
solubility
acetonitrile: 0.1 g/mL, clear
응용 분야
peptide synthesis
작용기
amine
저장 온도
2-8°C
SMILES string
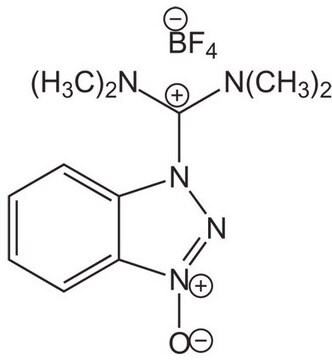
F[P-](F)(F)(F)(F)F.CN(C)C(\On1nnc2ccccc12)=[N+](/C)C
InChI
1S/C11H16N5O.F6P/c1-14(2)11(15(3)4)17-16-10-8-6-5-7-9(10)12-13-16;1-7(2,3,4,5)6/h5-8H,1-4H3;/q+1;-1
InChI key
UQYZFNUUOSSNKT-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Skin Sens. 1A
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
문서
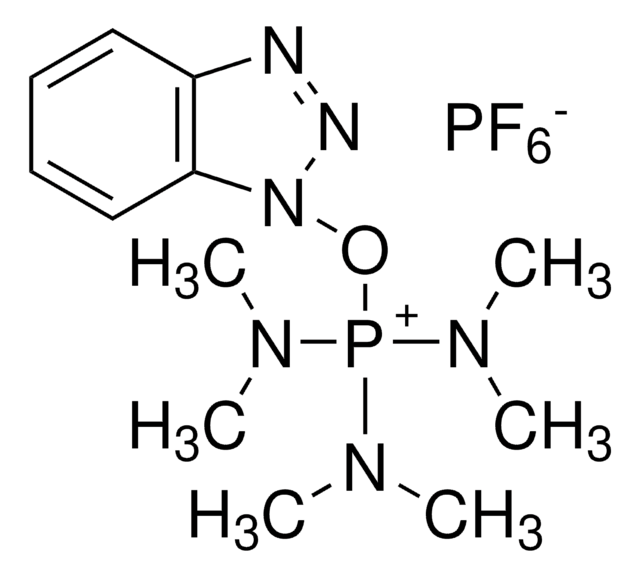
The special need of SPPS for rapid and highly efficient coupling reagents led to the development of several new reagents starting from BOP (Benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate).
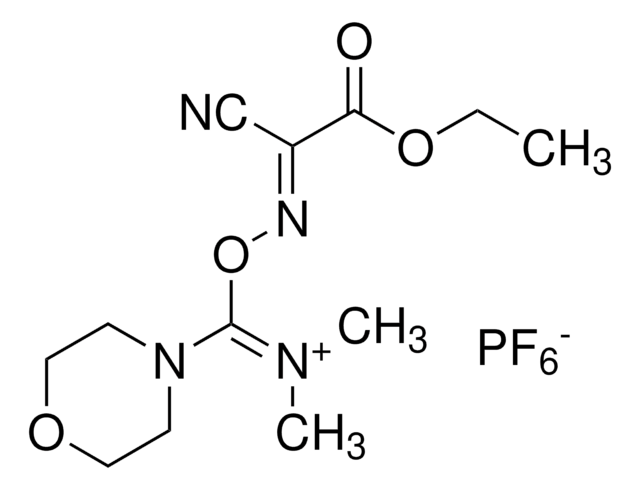
COMU is a non-explosive coupling agent suitable for solution phase & solid phase peptide synthesis. Its activity meets or exceeds that of HATU and its water-soluble by-product are easily removed.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.