추천 제품
일반 설명
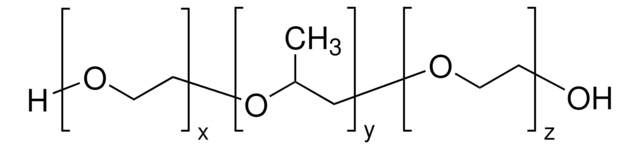
Detergents, such as non-ionic surfactants, are used in biomanufacturing processes for a variety of applications including viral inactivation and cell lysis. Detergent-mediated viral inactivation is widely used in multiple biotherapeutic production processes to ensure meeting regulatory requirements for viral safety.
Our SAFC portfolio of high-quality raw materials for use in biopharmaceutical processing withstands strict quality control procedures plus the documentation and expertise to help our customers meet requirements as defined by the M-Clarity Program.
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of downstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Ranging from non-GMP grades for low-risk application, to IPEC-PQG GMP for higher-risk applications, we have products covering all your manufacturing needs.
We offer a range of high quality critical raw materials plus the documentation and expertise to help our customers meet stringent regulatory requirements. Our SAFC portfolio of high-quality products withstands strict quality control procedures.
Our SAFC portfolio of high-quality raw materials for use in biopharmaceutical processing withstands strict quality control procedures plus the documentation and expertise to help our customers meet requirements as defined by the M-Clarity Program.
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of downstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Ranging from non-GMP grades for low-risk application, to IPEC-PQG GMP for higher-risk applications, we have products covering all your manufacturing needs.
We offer a range of high quality critical raw materials plus the documentation and expertise to help our customers meet stringent regulatory requirements. Our SAFC portfolio of high-quality products withstands strict quality control procedures.
애플리케이션
REACH compliantdetergent used in viral inactivation or cell lysis.
포장
1.08694.1000 / 1L / Glass bottle
1.08694.2500 / 2.5L / Glass bottle
1.08694.9025 / 25L / PE container
1.08694.2500 / 2.5L / Glass bottle
1.08694.9025 / 25L / PE container
법적 정보
DEVIRON is a registered trademark of Merck KGaA, Darmstadt, Germany
Emprove is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
469.4 °F - open cup
Flash Point (°C)
243 °C - open cup
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.