V403
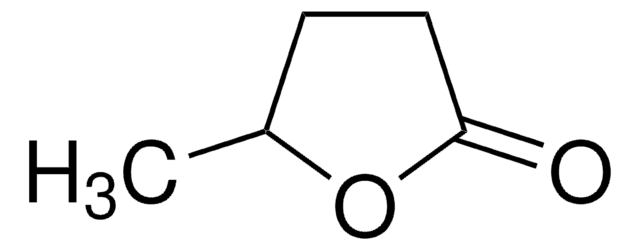
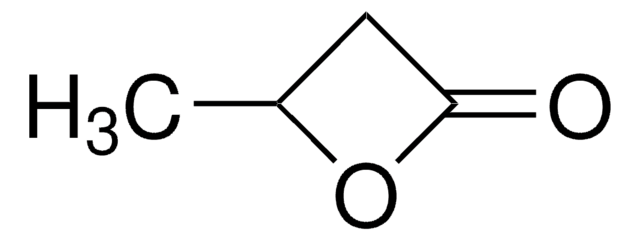
γ-Valerolactone
ReagentPlus®, 99%
동의어(들):
γ-Valerolactone, gamma-Valerolactone, γ-Methyl-γ-butyrolactone, 4,5-Dihydro-5-methyl-2(3H)-furanone, 4-Hydroxypentanoic acid lactone
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C5H8O2
CAS Number:
Molecular Weight:
100.12
Beilstein:
80420
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
vapor density
3.45 (vs air)
제품 라인
ReagentPlus®
분석
99%
refractive index
n20/D 1.432 (lit.)
bp
207-208 °C (lit.)
82-85 °C/10 mmHg (lit.)
mp
−31 °C (lit.)
density
1.05 g/mL at 25 °C (lit.)
SMILES string
CC1CCC(=O)O1
InChI
1S/C5H8O2/c1-4-2-3-5(6)7-4/h4H,2-3H2,1H3
InChI key
GAEKPEKOJKCEMS-UHFFFAOYSA-N
유전자 정보
human ... CYP1A2(1544)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
γ-valerolactone (GVL) is found naturally in fruits and often used as a fuel additive and food ingredient. It is prominently employed in the production of both energy and carbon-based products.
애플리케이션
γ-valerolactone (GVL) can be used as a green solvent:
- To transform lignocellulose into furfural using a solid acid catalyst, H-mordenite.
- To synthesize phosphatidylserine.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
204.8 °F - closed cup
Flash Point (°C)
96 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
γ-Valerolactone-a sustainable liquid for energy and carbon-based chemicals
Horvath I, et al.
Green Chemistry, 10(2), 238-242 (2008)
Highly efficient synthesis of phosphatidylserine in the eco-friendly solvent γ-valerolactone
Duan Z-Q and Hu Fei
Green Chemistry, 14(6), 1581-1583 (2012)
Jesse Q Bond et al.
Langmuir : the ACS journal of surfaces and colloids, 26(21), 16291-16298 (2010-06-02)
γ-Valerolactone (GVL) has been identified as a promising, sustainable platform molecule that can be produced from lignocellulosic biomass. The chemical flexibility of GVL has allowed the development of a variety of processes to prepare renewable fuels and chemicals. In the
Jean-Paul Lange et al.
Chemical communications (Cambridge, England), (33)(33), 3488-3490 (2007-08-19)
Methyl pentenoate, a promising Nylon intermediate, is produced in >95% yield via the transesterification of gamma-valerolactone, a bio-based intermediate, under catalytic distillation conditions.
Laureen J Marinetti et al.
Pharmacology, biochemistry, and behavior, 101(4), 602-608 (2012-02-22)
Gamma butyrolactone (GBL) is metabolized to gamma hydroxybutyrate (GHB) in the body. GHB is a DEA Schedule 1 compound; GBL is a DEA List 1 chemical. Gamma valerolactone (GVL) is the 4-methyl analog of GBL; GVL is metabolized to 4-methyl-GHB;
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.