T62804
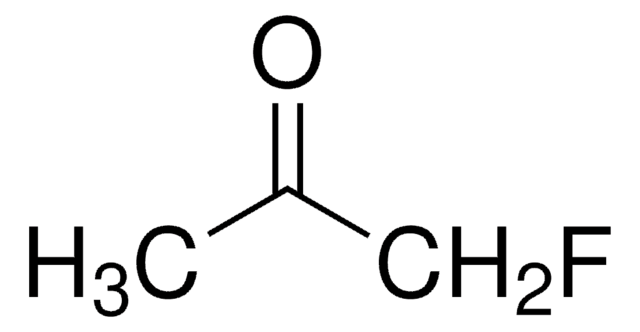
1,1,1-Trifluoroacetone
97%
동의어(들):
α,α,α-Trifluoroacetone, 1,1,1-Trifluoro-2-propanone, 3,3,3-Trifluoroacetone, Methyl trifluoromethyl ketone, Trifluoromethyl methyl ketone
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
CH3COCF3
CAS Number:
Molecular Weight:
112.05
Beilstein:
1748614
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
vapor pressure
13.62 psi ( 20 °C)
Quality Level
분석
97%
refractive index
n20/D 1.3 (lit.)
bp
22 °C (lit.)
density
1.252 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
CC(=O)C(F)(F)F
InChI
1S/C3H3F3O/c1-2(7)3(4,5)6/h1H3
InChI key
FHUDAMLDXFJHJE-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Used in a synthesis of 2-trifluoromethyl-7-azaindoles starting with 2,6-dihalopyridines. The derived chiral imine was used to prepare enantiopure α-trifluoromethyl alanines and diamines via a Strecker reaction followed by either nitrile hydrolysis or reduction.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Flam. Liq. 1 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
-22.0 °F - closed cup
Flash Point (°C)
-30 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves
이미 열람한 고객
Florent Huguenot et al.
The Journal of organic chemistry, 71(18), 7075-7078 (2006-08-26)
Diastereomerically pure alpha-trifluoromethyl alpha-amino nitriles obtained by Strecker-type reactions from chiral CF3 imines and iminium proved to be very attractive versatile intermediates for the synthesis of various alpha-trifluoromethyl amino compounds. From these synthons, both enantiomers of alpha-trifluoromethyl alanine, trifluoromethyl 1,2-diamines
Synthesis, 251-251 (2007)
Emre Kinaci et al.
Polymers, 12(9) (2020-09-20)
In this investigation, the terminal double bonds of the side chain epoxidized cardanol glycidyl ether (SCECGE) molecule were further epoxidized in the presence of Oxone® (potassium peroxomonosulfate) and fluorinated acetone. Regular methods for the double bond epoxidation are not effective
Sheida Esmaielzadeh et al.
Acta chimica Slovenica, 63(2), 351-362 (2016-06-23)
Some cobalt(III) complexes with a potentially tetradentate unsymmetrical NNOS Schiff base ligand have been synthesized and characterized using IR, 1HNMR, UV-Vis spectroscopy and elemental analysis. The equilibrium constants were measured spectrophotometrically for 1:1 adduct formation of the cobalt(III) complexes with
Jobst Liebau et al.
The Journal of biological chemistry, 295(29), 9868-9878 (2020-05-22)
Fold-switch pathways remodel the secondary structure topology of proteins in response to the cellular environment. It is a major challenge to understand the dynamics of these folding processes. Here, we conducted an in-depth analysis of the α-helix-to-β-strand and β-strand-to-α-helix transitions
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.