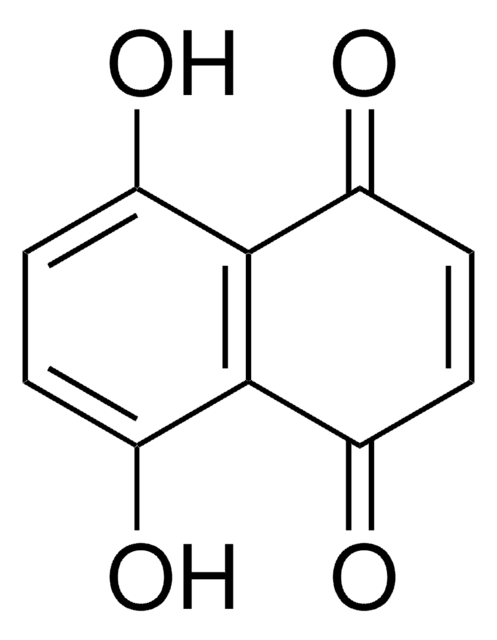
추천 제품
분석
97%
mp
161-163 °C (lit.)
SMILES string
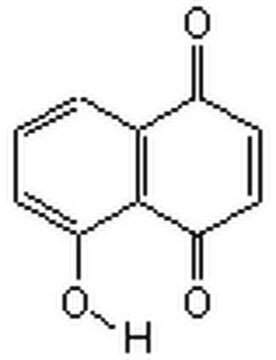
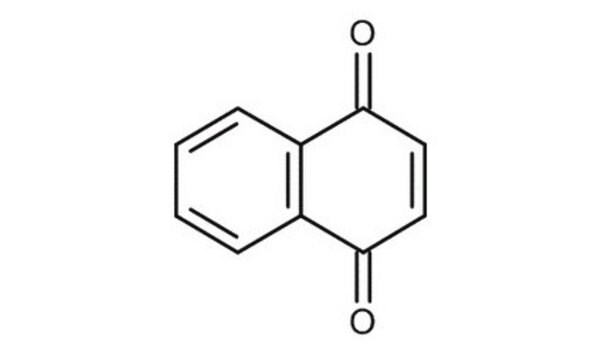
Oc1cccc2C(=O)C=CC(=O)c12
InChI
1S/C10H6O3/c11-7-4-5-9(13)10-6(7)2-1-3-8(10)12/h1-5,12H
InChI key
KQPYUDDGWXQXHS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
5-Hydroxy-1,4-naphthoquinone (juglone) can be used as a starting material for the synthesis of:
Juglone can also be used as a dienophile in the Diels–Alder reaction for the synthesis of variety of C-aryl glycosides.
- Trypanocidal drugs.
- Tacrine-naphthoquinone hybrids with potential application in the treatment of Alzheimer′s disease.
- Juglone-based electroactive polymer, poly(5-hydroxy-1,4-naphthoquinone-co-5-hydroxy-3-thioacetic acid-1,4-naphthoquinone), for the electrochemical detection of DNA hybridization.
- Ent-nocardione A, naturally-occurring tyrosine phosphatase inhibitor.
Juglone can also be used as a dienophile in the Diels–Alder reaction for the synthesis of variety of C-aryl glycosides.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
Multitarget drug design strategy: quinone-tacrine hybrids designed to block amyloid-β aggregation and to exert anticholinesterase and antioxidant effects.
Nepovimova E, et al.
Journal of medicinal chemistry, 57(20), 8576-8589 (2014)
Application of an Enyne Metathesis/Diels-Alder Cycloaddition Sequence: A New Versatile Approach to the Syntheses of C-Aryl Glycosides and Spiro-C-Aryl Glycosides.
Subrahmanyam AV, et al.
Chemistry?A European Journal, 16(28), 8545-8556 (2010)
2-and 3-substituted 1, 4-naphthoquinone derivatives as subversive substrates of trypanothione reductase and lipoamide dehydrogenase from trypanosoma c ruzi: synthesis and correlation between redox cycling activities and in vitro cytotoxicity.
Salmon-Chemin L, et al.
Journal of Medicinal Chemistry, 44(4), 548-565 (2001)
Study of the DNA hybridization transduction behavior of a quinone-containing electroactive polymer by cyclic voltammetry and electrochemical impedance spectroscopy.
Piro B, et al.
Journal of Electroanalytical Chemistry, 577(1), 155-165 (2005)
Formal Radical Cyclization onto Benzene Rings: A General Method and Its Use in the Synthesis of ent-Nocardione A.
Clive DLJ, et al.
The Journal of Organic Chemistry, 69(10), 3282-3293 (2004)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.