추천 제품
분석
99%
양식
liquid
refractive index
n20/D 1.453 (lit.)
bp
139-140 °C (lit.)
mp
−19 °C (lit.)
density
0.948 g/mL at 20 °C
0.949 g/mL at 25 °C (lit.)
SMILES string
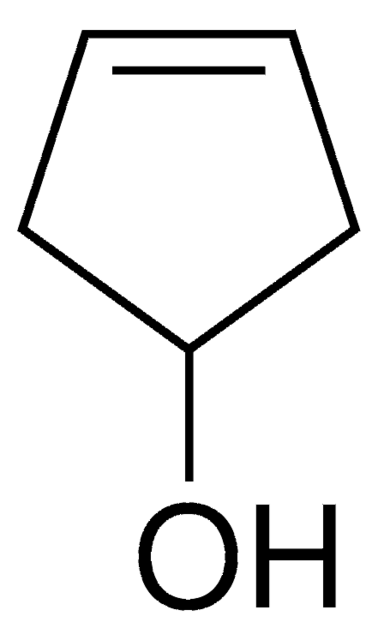
OC1CCCC1
InChI
1S/C5H10O/c6-5-3-1-2-4-5/h5-6H,1-4H2
InChI key
XCIXKGXIYUWCLL-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Cyclopentanol can be used as:
- An alkylating agent in the preparation of alkylated aromatic compounds using Fe3+-montmorillonite catalyst via Friedel–Crafts alkylation reaction.
- A reactant in the acylation of alcohols with an acid anhydride or acid chloride.
- A substrate in the synthesis of high-density polycyclic aviation fuel by the Guerbet reaction.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
123.8 °F - closed cup
Flash Point (°C)
51 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Three-component coupling reactions of silyl glyoxylates, vinyl Grignard reagent, and nitroalkenes: an efficient, highly diastereoselective approach to nitrocyclopentanols.
Gregory R Boyce et al.
Angewandte Chemie (International ed. in English), 49(47), 8930-8933 (2010-10-16)
Marie Bøjstrup et al.
Organic & biomolecular chemistry, 3(9), 1738-1745 (2005-04-29)
Four aminocyclopentanols, as mimics of putative intermediates in the hydrolysis of alpha-d-galactosides, have been synthesized through a number of stereoselective transformations using the cis-fused cyclopentane-1,4-lactone (1R, 5S, 7R, 8R)-7,8-dihydroxy-2-oxabicyclo[3.3.0]oct-3-one as a chiral building block. The compounds were tested towards various
Gregory R Boyce et al.
Organic letters, 14(2), 652-655 (2012-01-13)
The three-component coupling of Mg acetylides, silyl glyoxylates, and nitroalkenes results in a highly diastereoselective Kuwajima-Reich/vinylogous Michael cascade that provides tetrasubstituted silyloxyallene products. The regio- and diastereoselectivity were studied using DFT calculations. These silyloxyallenes were converted to cyclopentenols and cyclopentitols
E Leroy et al.
Organic letters, 1(5), 775-777 (2000-05-24)
[reaction: see text] (1S,2S,3S,4R,5R)-4-amino-5-(hydroxymethyl)cyclopentane-1,2,3-triol 1 is prepared stereoselectively from D-lyxose and displays anomer-selective inhibition for beta-galactosidase (Ki = 3.0 x 10(-6) M) and beta-glucosidase (Ki = 1.5 x 10(-7) M), over alpha-galactosidase (Ki = 2.3 x 10(-5) M) and alpha-glucosidase
R N Hanson et al.
International journal of radiation applications and instrumentation. Part A, Applied radiation and isotopes, 38(8), 641-645 (1987-01-01)
Radioiododestannylations was employed to prepare a series of four specifically labeled thienyl alcohols: 1-(5-iodo-2-thienyl)-cyclopentan-1-ol and -cyclohexan-1-ol; 17 alpha-(5-iodo-2-thienyl)-17 beta-estradiol and -estradiol-3-O-methyl ether. The method utilized 5-(trimethylstannyl)thienyl intermediates which had been prepared in good yields from 2,5-bis(trimethylstannyl)thiophene and the appropriate cyclic
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.