추천 제품
Quality Level
분석
97%
mp
59-62 °C (lit.)
작용기
carboxylic acid
ketone
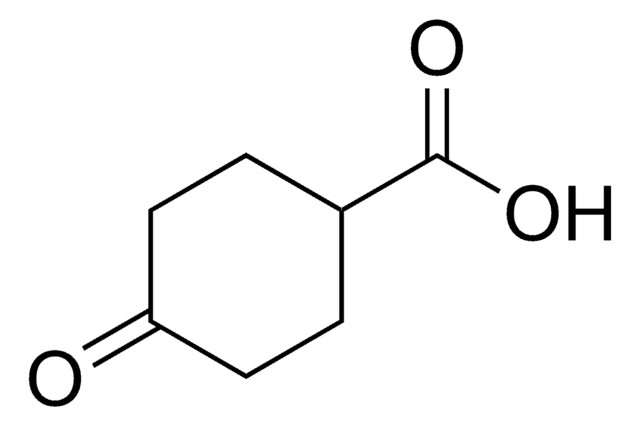
SMILES string
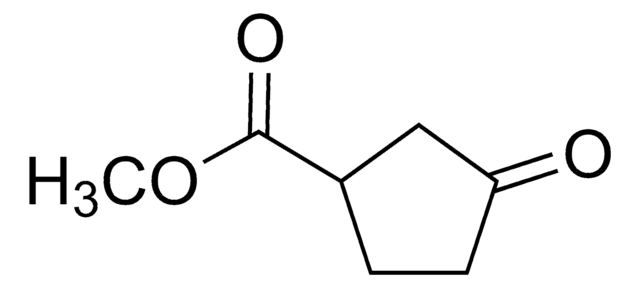
OC(=O)C1CCC(=O)C1
InChI
1S/C6H8O3/c7-5-2-1-4(3-5)6(8)9/h4H,1-3H2,(H,8,9)
InChI key
RDSNBKRWKBMPOP-UHFFFAOYSA-N
일반 설명
3-Oxo-1-cyclopentanecarboxylic acid , also known as 3-oxocyclopentanecarboxylic acid, is a keto acid derivative. It undergoes Curtius rearrangement with diphenyl phosphoryl azide and triethylamine in tert-butanol to form the corresponding boc-protected 1-(3-oxo)urea derivative.
애플리케이션
3-Oxo-1-cyclopentanecarboxylic acid may be used in the preparation of 3-hydroxycyclopentanecarboxylic acid via hydrogenation.
Substrate used in a study of biohydroxylation with mutants of cytochrome P450 BM-3.
법적 정보
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
Dieter F Münzer et al.
Chemical communications (Cambridge, England), (20), 2597-2599 (2005-05-19)
Substrate engineered, achiral carboxylic acid derivative was biohydroxylated with various mutants of cytochrome P450 BM-3 to give two out of the four possible diastereoisomers in high de and ee. The BM-3 mutants exhibit up to 9200 total turnovers for hydroxylation
Studies of Configuration. V. The Preparation and Configuration of cis-3-Methoxycyclopentanecarboxylic Acid.
Noyce D and Fessenden J.
The Journal of Organic Chemistry, 24(5), 715-717 (1959)
Boc-protected 1-(3-oxocycloalkyl) ureas via a one-step Curtius rearrangement: mechanism and scope.
Sun X, et al.
Tetrahedron Letters, 55(4), 842-844 (2014)
문서
Phase I biotransformation reactions introduce or expose functional groups on the drug with the goal of increasing the polarity of the compound. Although Phase I drug metabolism occurs in most tissues, the primary and first pass site of metabolism occurs during hepatic circulation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.