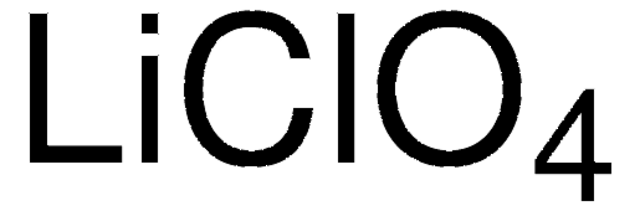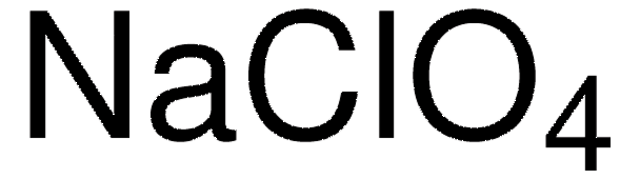추천 제품
Grade
anhydrous
battery grade
Quality Level
분석
≥99.9% trace metals basis
형태
powder
환경친화적 대안 제품 특성
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
불순물
≤1000 ppm (trace metals analysis)
pH
6.0-7.5 (25 °C, 5%, aq.sol.)
mp
236 °C (lit.)
solubility
H2O: 59.8 g/dL at 25 °C
음이온 미량물
chloride (Cl-): ≤30 ppm
sulfate (SO42-): ≤10 ppm
양이온 미량물
Fe: ≤5 ppm
heavy metals: ≤10 ppm
응용 분야
battery manufacturing
환경친화적 대안 카테고리
SMILES string
[Li+].[O-]Cl(=O)(=O)=O
InChI
1S/ClHO4.Li/c2-1(3,4)5;/h(H,2,3,4,5);/q;+1/p-1
InChI key
MHCFAGZWMAWTNR-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Anhydrous lithium perchlorate is a white-to-colorless crystalline salt. It is hygroscopic and deliquescent and usually stored under inert atmosphere. It is highly soluble in water and soluble in a variety of organic solvents including alcohols, acetone, acetonitrile, ethyl acetate, ethers, carbonates, and other polar organic solvents. Lithium perchlorate is a strong oxidizing agent.
Industrially, lithium perchlorate is manufactured in several ways. Most commonly, it is prepared from sodium perchlorate through a metathesis reaction with lithium chloride or lithium carbonate. Lithium perchlorate can also be prepared by direct electrochemical oxidation of lithium chloride or by reacting lithium carbonate with perchloric acid. The hydrate can be dried either by highly controlled heating or by displacing water with volatile amines, which are removed by drying under vacuum.
Industrially, lithium perchlorate is manufactured in several ways. Most commonly, it is prepared from sodium perchlorate through a metathesis reaction with lithium chloride or lithium carbonate. Lithium perchlorate can also be prepared by direct electrochemical oxidation of lithium chloride or by reacting lithium carbonate with perchloric acid. The hydrate can be dried either by highly controlled heating or by displacing water with volatile amines, which are removed by drying under vacuum.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Click here for more information.
애플리케이션
The primary application of lithium perchlorate is as an electrolytic salt in lithium-ion batteries. Many of the early, now-famous reports of lithium batteries used lithium perchlorate dissolved in polar organics as the electrolyte and the salt remains popular because of its high solubility, electrochemical stability, and low cost. In the search for solid electrolytes, lithium perchlorate (5-12 wt%) is often added to polyethylene oxide (PEO) and composited with ceramic nanoparticles like LLZO and LATP .
Researchers also use lithium perchlorate as an electrolytic salt in aqueous media when testing electrocatalysts. For example, recent experiments improving the electrochemical reduction of nitrogen over TiO2 nanoparticles or gold nanoparticles use aqueous lithium perchlorate as the electrolyte.
Researchers also use lithium perchlorate as an electrolytic salt in aqueous media when testing electrocatalysts. For example, recent experiments improving the electrochemical reduction of nitrogen over TiO2 nanoparticles or gold nanoparticles use aqueous lithium perchlorate as the electrolyte.
포장
100g in poly bottle
500g in poly bottle
500g in poly bottle
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1A - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Electrochemical and In Situ X?Ray Diffraction Studies of Lithium Intercalation in Lix CoO2
Reimers, J.N., et al.
Journal of the Electrochemical Society, 139, 2091-2091 (1992)
The spinel phase of lithium manganese oxide (LiMn2O4) as a cathode in secondary lithium cells
Tarascon, J.M., et al.
Journal of the Electrochemical Society, 138, 2859-2864 (1991)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.