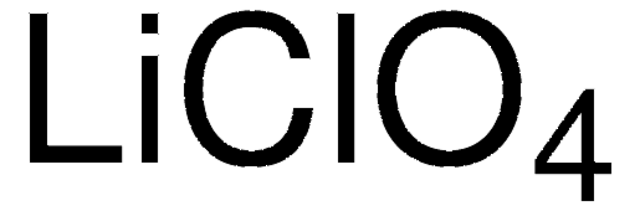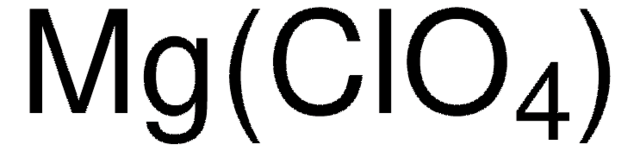추천 제품
Grade
ACS reagent
분석
99.99% trace metals basis
형태
granular
반응 적합성
reagent type: oxidant
환경친화적 대안 제품 특성
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
불순물
≤0.005% insolubles
<100 ppm total metallic impurities
pH
6.0-7.5
mp
236 °C (lit.)
solubility
H2O: 106.4 g/L at 20 °C
음이온 미량물
chloride (Cl-): ≤0.003%
sulfate (SO42-): ≤0.001%
환경친화적 대안 카테고리
SMILES string
[Li+].[O-]Cl(=O)(=O)=O
InChI
1S/ClHO4.Li/c2-1(3,4)5;/h(H,2,3,4,5);/q;+1/p-1
InChI key
MHCFAGZWMAWTNR-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Lithium perchlorate is a white salt that easily dissolves in water. It is commonly employed as an electrolytic salt in batteries due to its excellent electrical impedance, conductivity, hygroscopicity, and anodic stability properties. These characteristics are crucial for its role in battery applications. In addition, it is as a strong oxidizing agent and hence is used as an oxidizer in solid rocket propellants and organic reactions.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Click here for more information.
애플리케이션
Lithium perchlorate can be used:
- As a precursor to prepare solid polymer electrolytes for rechargeable Li-ion batteries.
- As an oxidizer to prepare polymer-based solid propellants.
- To fabricate cobalt sulfide (CoS)-based counter electrode for dye-sensitized solar cells(DSSC).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1A - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Shokaku Kim et al.
Organic letters, 4(21), 3735-3737 (2002-10-12)
[reaction: see text] N-Acyliminium cation of prolines was efficiently generated to accumulate in an undivided cell at 0 degrees C by an anodic oxidation of N-acylprolines or alpha'-phenylsulfanylated N-acylproline derivatives in a lithium perchlorate/nitromethane solution. The iminium cation intermediates gave
L H Sim et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 76(3-4), 287-292 (2010-05-07)
The interaction behaviours between components of polyacrylate (PAc)/poly(ethylene oxide) (PEO) and lithium perchlorate (LiClO(4)) were investigated in detail by Attenuated Total Reflectance (ATR)-Fourier Transformed Infrared (FTIR) spectroscopy. Solution cast films of the PAc/PEO and PAc/PEO/LiClO(4) were examined. No obvious shifting
Natalia Varaksa et al.
Proceedings of the National Academy of Sciences of the United States of America, 99(8), 5012-5017 (2002-04-18)
The adsorption of the trigonal connector, 1,3,5-tris[10-(3-ethylthiopropyl)dimethylsilyl-1,10-dicarba-closo-decaboran-1-yl]benzene (1), from acetonitrile/0.1 M LiClO(4) on the surface of mercury at potentials ranging from +0.3 to -1.4 V (vs. aqueous Ag/AgCl/1 M LiCl) was examined by voltammetry, Langmuir isotherms at controlled potentials, and
Witold Darlewski et al.
Journal of hazardous materials, 175(1-3), 460-467 (2009-11-17)
A process of dibutyl sulphide (DBS) electro-oxidation using electrolysis and cyclic voltamperometry was investigated in water-methanol solution using different electrodes (platinum, boron doped diamond, graphite and glassy carbon). Obtained results indicate that the DBS electro-oxidation process is irreversible in voltamperometric
Francisco Palacios et al.
The Journal of organic chemistry, 67(7), 2131-2135 (2002-04-02)
Functionalized keto-enamines 6 were obtained by nucleophilic addition of enol ethers to the imine moiety of 2-azadienes derived from dehydroaspartic esters 4. Reactions of 2-azadiene 4c containing three electron-withdrawing substituents (CO(2)R) with enol ethers 5 in the presence of lithium
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.