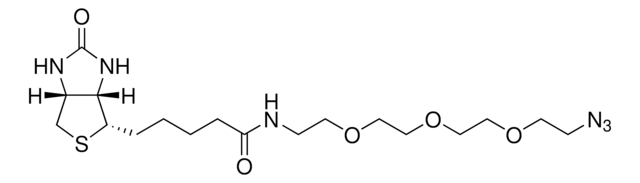
907367
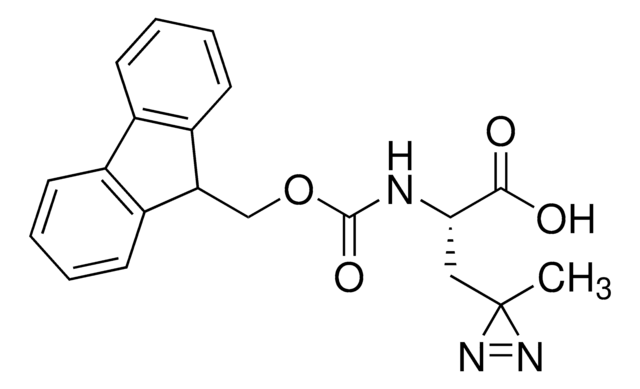
Fmoc-L-photo-methionine
≥95%, for peptide synthesis
동의어(들):
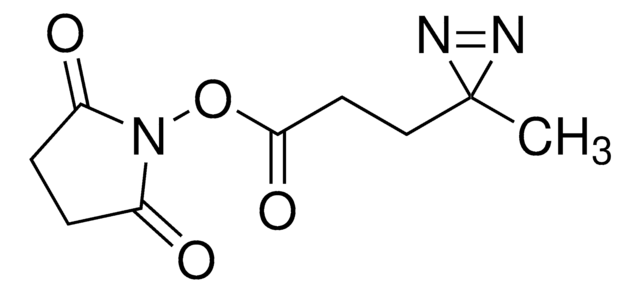
(S)-2-(((9H-Fluoren-9-yl)methoxy)carbonylamino)-4-(3-methyl-3H-diazirin-3-yl)butanoic acid, (S)-2-(Fmoc-amino)-4-(3H-diazirin-3-yl)pentanoic acid, Diazirine amino acid, Photo-Met, Photo-crosslinking amino acid, Photoprobe building block
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C21H21N3O4
CAS Number:
Molecular Weight:
379.41
MDL number:
UNSPSC 코드:
12352209
NACRES:
NA.22
추천 제품
제품명
Fmoc-L-photo-methionine, ≥95%
분석
≥95%
양식
powder
반응 적합성
reaction type: Fmoc solid-phase peptide synthesis
응용 분야
peptide synthesis
작용기
Fmoc
저장 온도
−20°C
SMILES string
N([C@@H](CCC4(N=N4)C)C(=O)O)C(=O)OCC1c2c(cccc2)c3c1cccc3
InChI key
QKMQUEIDJLPYHS-SFHVURJKSA-N
애플리케이션
Fmoc-L-photo-methionine is a diazirine-containing, Fmoc-protected methionine amino acid and multifunctional photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An unprotected version is also available as 907375.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
기타 정보
Developing diazirine-based chemical probes to identify histone modification ′readers′ and ′erasers ′
Proteome profiling reveals potential cellular targets of staurosporine using a clickable cell-permeable probe
Covalent Capture of Phospho-Dependent Protein Oligomerization by Site-Specific Incorporation of a Diazirine Photo-Cross-Linker
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Proteome profiling reveals potential cellular targets of staurosporine using a clickable cell-permeable probe
Covalent Capture of Phospho-Dependent Protein Oligomerization by Site-Specific Incorporation of a Diazirine Photo-Cross-Linker
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
관련 제품
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Covalent capture of phospho-dependent protein oligomerization by site-specific incorporation of a diazirine photo-cross-linker.
Miquel Vila-Perelló et al.
Journal of the American Chemical Society, 129(26), 8068-8069 (2007-06-15)
Haibin Shi et al.
Chemical communications (Cambridge, England), 47(40), 11306-11308 (2011-09-17)
A clickable, affinity-based probe (AfBP), which was modified from staurosporine (a natural product kinase inhibitor), has been synthesized and used in situ for activity-based proteome profiling of potential cellular targets of staurosporine in HepG2 cancer cells.
Developing diazirine-based chemical probes to identify histone modification 'readers' and 'erasers'.
Tangpo Yang et al.
Chemical science, 6(2), 1011-1017 (2015-02-01)
Post translational modifications (PTMs, e.g., phosphorylation, acetylation and methylation) of histone play important roles in regulating many fundamental cellular processes such as gene transcription, DNA replication and damage repair. While 'writer' and 'eraser' enzymes modify histones by catalyzing the addition
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 907367-100MG | 4022536044583 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.