907391
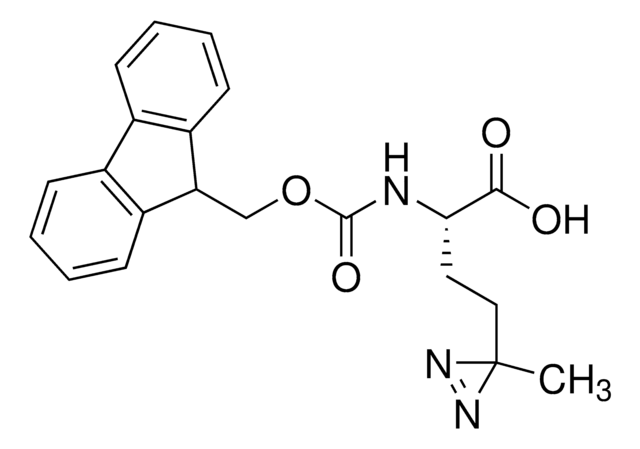
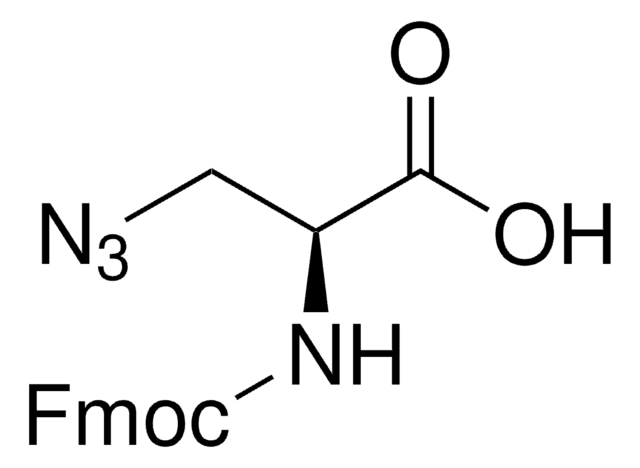
Fmoc-L-photo-leucine
≥98%
동의어(들):
(S)-2-(((9H-Fluoren-9-yl)methoxy)carbonylamino)-3-(3-methyl-3H-diazirin-3-yl)propanoic acid, (S)-2-(Fmoc-amino)-3-(3H-diazirin-3-yl)butanoic acid, Photo-Leu, Photo-crosslinking amino acid, Photoprobe building block
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C20H19N3O4
CAS Number:
Molecular Weight:
365.38
UNSPSC 코드:
12352209
NACRES:
NA.22
추천 제품
분석
≥98%
형태
powder
반응 적합성
reaction type: Fmoc solid-phase peptide synthesis
응용 분야
peptide synthesis
작용기
Fmoc
저장 온도
2-8°C
애플리케이션
Fmoc-L-Photo-Leucine is a diazirine-containing, Fmoc-protected leucine amino acid and multifunctional photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An unprotected version is also available as 907278.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
기타 정보
Synthesis of a polymyxin derivative for photolabeling studies in the gram-negative bacterium Escherichia coli
Developing diazirine-based chemical probes to identify histone modification ′readers′ and ′erasers′
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Developing diazirine-based chemical probes to identify histone modification ′readers′ and ′erasers′
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
관련 제품
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Benjamin van der Meijden et al.
Journal of peptide science : an official publication of the European Peptide Society, 21(3), 231-235 (2015-02-03)
The antimicrobial activity of polymyxins against Gram-negative bacteria has been known for several decades, but the mechanism of action leading to cell death has not been fully explored. A key step after binding of the antibiotic to lipopolysaccharide (LPS) exposed
Aleš Marek et al.
Journal of the American Society for Mass Spectrometry, 25(5), 778-789 (2014-02-20)
Gas-phase dissociations were investigated for several peptide ions containing the Gly-Leu* N-terminal motif where Leu* was a modified norleucine residue containing the photolabile diazirine ring. Collisional activation of gas-phase peptide cations resulted in facile N₂ elimination that competed with backbone
Jay M Janz et al.
Journal of the American Chemical Society, 133(40), 15878-15881 (2011-09-13)
Cell surface heptahelical G protein-coupled receptors (GPCRs) mediate critical cellular signaling pathways and are important pharmaceutical drug targets. (1) In addition to traditional small-molecule approaches, lipopeptide-based GPCR-derived pepducins have emerged as a new class of pharmaceutical agents. (2, 3)
Developing diazirine-based chemical probes to identify histone modification 'readers' and 'erasers'.
Tangpo Yang et al.
Chemical science, 6(2), 1011-1017 (2015-02-01)
Post translational modifications (PTMs, e.g., phosphorylation, acetylation and methylation) of histone play important roles in regulating many fundamental cellular processes such as gene transcription, DNA replication and damage repair. While 'writer' and 'eraser' enzymes modify histones by catalyzing the addition
En-Wei Lin et al.
Bioconjugate chemistry, 25(10), 1902-1909 (2014-10-16)
A photoactivated, site-selective conjugation of poly(ethylene glycol) (PEG) to the glutathione (GSH) binding pocket of glutathione S-transferase (GST) is described. To achieve this, a GSH analogue (GSH-BP) was designed and chemically synthesized with three functionalities: (1) the binding affinity of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.