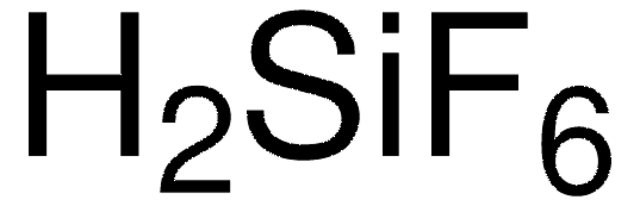추천 제품
양식
liquid
색상
colorless
pH
3-5
저장 온도
15-25°C
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Improved HF Buffer System with stabilized HF activity - selective solvent for SiO2 used in semiconductor technology of planar passivated devices - transistors, integrated circuits, diodes, rectifiers, SCR, MOS, FET
Advantages:
Buffer HF improved is an idealized buffer preparation characterized by a high buffer index and an optimized, uniform oxide-etch rate. The composition of buffer HF improved is precisely controlled by HF activity measurements and electrometric pH. The mass balance corresponds essentially to (HF) + (F) + 2(HF2) for a two-ligand mononuclear complex and the charge balance is (H+) - (F) + (HF2- ). The HF activity is maintained constant through the specific equilibrium constant which regulates the equilibrium reaction between fluoride, bifluoride, and HF buffer components. A second equilibrium constant participates in the regulation of the hydronium in concentration of pH.
Buffer HF improved is produced and analyzed to be essentially free of impurities. Nitrate ions, a common impurity causing stains on diffused silicon surfaces, are specifically removed. Heavy metal impurities, which can lead to degradation of device characteristics, are rigidly controlled under manufacturing process specifications.
Advantages:
- Ready-to-use - Economical
- HF activity buffer stabilized
- Excellent process reproducibility
- Does not undercut masked oxide
- Will not stain diffused silicon surfaces
- Avoids contamination on silicon surfaces
- Photoresist coating unaffected
Buffer HF improved is an idealized buffer preparation characterized by a high buffer index and an optimized, uniform oxide-etch rate. The composition of buffer HF improved is precisely controlled by HF activity measurements and electrometric pH. The mass balance corresponds essentially to (HF) + (F) + 2(HF2) for a two-ligand mononuclear complex and the charge balance is (H+) - (F) + (HF2- ). The HF activity is maintained constant through the specific equilibrium constant which regulates the equilibrium reaction between fluoride, bifluoride, and HF buffer components. A second equilibrium constant participates in the regulation of the hydronium in concentration of pH.
Buffer HF improved is produced and analyzed to be essentially free of impurities. Nitrate ions, a common impurity causing stains on diffused silicon surfaces, are specifically removed. Heavy metal impurities, which can lead to degradation of device characteristics, are rigidly controlled under manufacturing process specifications.
애플리케이션
Use of Buffer HF improved:
Buffer HF improved dissolves silica films (both thermally grown and silane SiO2) produced on the surfaces of silicon and exposed by photolithography. It also is capable of dissolving doped silica films such as phosphosilica and borosilica glasses as formed in semiconductor processing.
The overall chemical reaction is: 4HF + SiO2 SiF4 + 2H2O
For trouble-free operation Buffer HF improved is recommended in the new technologies for manufacture of semiconductor planar and mesa devices. It is compatible with both negative and positive photoresists. Excellent results with good reproducibility are simple to achieve without undercutting marked oxides, surface staining or device degradation by metallic impurities.
Buffer HF improved dissolves silica films (both thermally grown and silane SiO2) produced on the surfaces of silicon and exposed by photolithography. It also is capable of dissolving doped silica films such as phosphosilica and borosilica glasses as formed in semiconductor processing.
The overall chemical reaction is: 4HF + SiO2 SiF4 + 2H2O
For trouble-free operation Buffer HF improved is recommended in the new technologies for manufacture of semiconductor planar and mesa devices. It is compatible with both negative and positive photoresists. Excellent results with good reproducibility are simple to achieve without undercutting marked oxides, surface staining or device degradation by metallic impurities.
제조 메모
Instructions:
Most practical oxide passivation layers range from 2000 Å to 5000 Å in thickness, and good results are obtained by exposure in Buffer HF improved for 2 to 5 minutes at room temperature. Exposure time may be decreased or increased if necessary. Buffer HF improved should be rinsed off with deionized water. The high buffer index of Buffer HF improved permits repeated use of the buffer at fixed exposure time. For faster etch rate (approx. 2X) use Buffer HF improved at 35 °C.
Most practical oxide passivation layers range from 2000 Å to 5000 Å in thickness, and good results are obtained by exposure in Buffer HF improved for 2 to 5 minutes at room temperature. Exposure time may be decreased or increased if necessary. Buffer HF improved should be rinsed off with deionized water. The high buffer index of Buffer HF improved permits repeated use of the buffer at fixed exposure time. For faster etch rate (approx. 2X) use Buffer HF improved at 35 °C.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Eye Dam. 1 - Skin Corr. 1A
Storage Class Code
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
문서
Professor Gogotsi and Dr. Shuck introduce MXenes: a promising family of two-dimensional materials with a unique combination of high conductivity, hydrophilicity, and extensive tunability.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.