798746
Exo-4-anisole Kwon [2.2.1] bicyclic phosphine
동의어(들):
Exo-4-Methoxyphenyl Kwon [2.2.1] Bicyclic Phosphine, (1S,4S,5R)-5-(4-methoxyphenyl)-2-tosyl-2-aza-5-phosphabicyclo[2.2.1]heptane
About This Item
추천 제품
형태
powder
Quality Level
mp
85-90 °C
작용기
phosphine
sulfonamide
SMILES string
O=S(N1C[C@@]2([H])[P@](C3=CC=C(OC)C=C3)C[C@]1([H])C2)(C4=CC=C(C)C=C4)=O
InChI
1S/C19H22NO3PS/c1-14-3-9-19(10-4-14)25(21,22)20-12-18-11-15(20)13-24(18)17-7-5-16(23-2)6-8-17/h3-10,15,18H,11-13H2,1-2H3/t15-,18-,24?/m0/s1
InChI key
RMFZMNUGIFSNIE-YPAHSFBSSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
기타 정보
Technology Spotlight- Kwon Phosphines: P-Chiral Monodentate Phosphines from Hydroxyproline
Aldrichimica Acta Review- Nucleophilic Chiral Phosphines: Powerful and Versatile Catalysts for Asymmetric Annulations
관련 제품
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
문서
Chiral phosphines have been the staple ligands for asymmetric transition metal catalysis and more recently operate as catalysts in organic phosphinocatalysis.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![Endo-4-Methoxyphenyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/404/012/38bdf2c6-e120-483d-8c3c-8fa3b328963c/640/38bdf2c6-e120-483d-8c3c-8fa3b328963c.png)
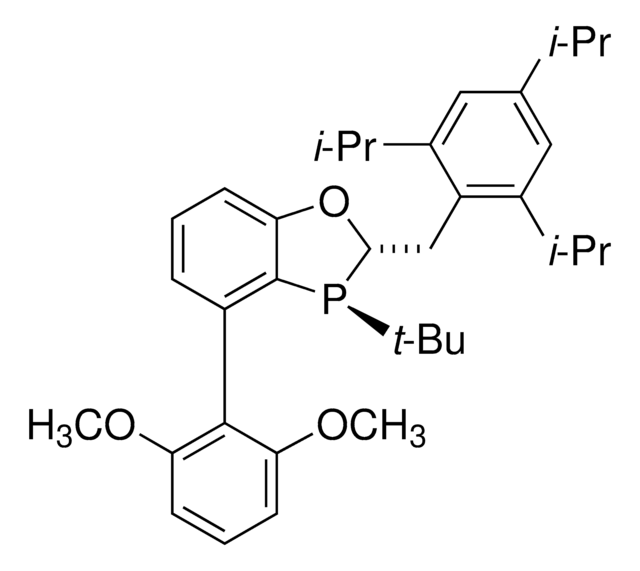
![Endo-1-Naphthyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/170/551/a39b471a-5427-43d5-9420-111b638ec1ac/640/a39b471a-5427-43d5-9420-111b638ec1ac.png)
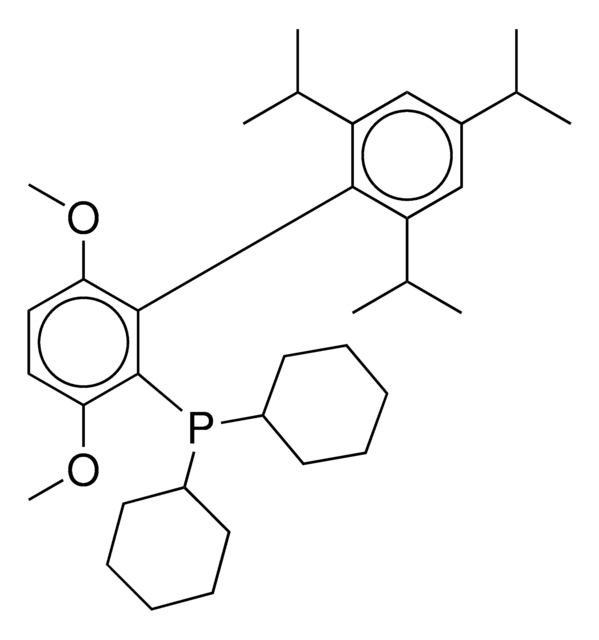
![Exo-Phenyl Kwon [2.2.1] Bicyclic Phosphine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/477/026/5255f657-4af5-47da-9839-86b94d92129f/640/5255f657-4af5-47da-9839-86b94d92129f.png)
![(3aR,8aR)-(−)-(2,2-Dimethyl-4,4,8,8-tetraphenyl-tetrahydro-[1,3]dioxolo[4,5-e][1,3,2]dioxaphosphepin-6-yl)dimethylamine 96%](/deepweb/assets/sigmaaldrich/product/structures/218/795/c536ff4e-370b-48fc-b22a-4120030fbfbb/640/c536ff4e-370b-48fc-b22a-4120030fbfbb.png)
![Endo-Phenyl Kwon [2.2.1] Bicyclic Phosphine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/207/060/39f2b621-f484-49c3-b692-cdb610e8c517/640/39f2b621-f484-49c3-b692-cdb610e8c517.png)
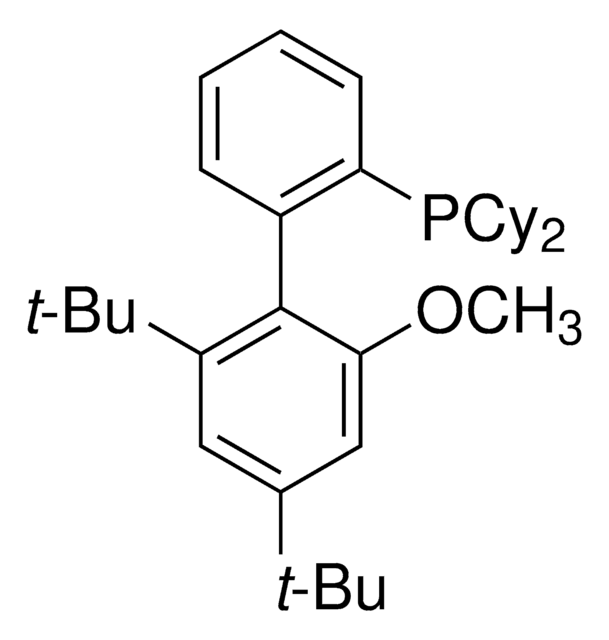
![Exo-2-Naphthyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/324/907/a6c29ce5-9be1-4585-9bfb-6dca8272f6e4/640/a6c29ce5-9be1-4585-9bfb-6dca8272f6e4.png)

