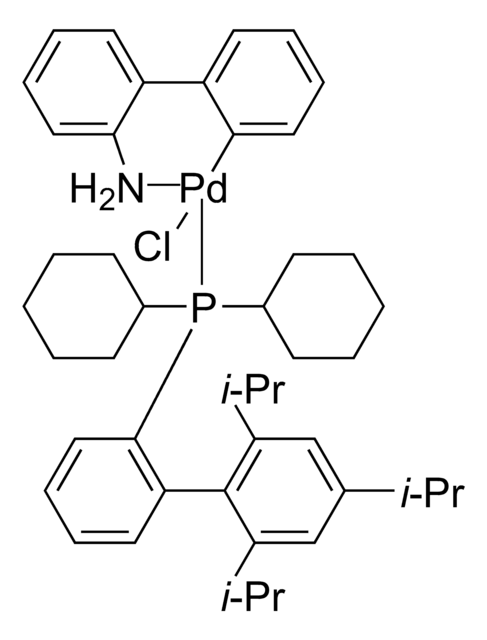
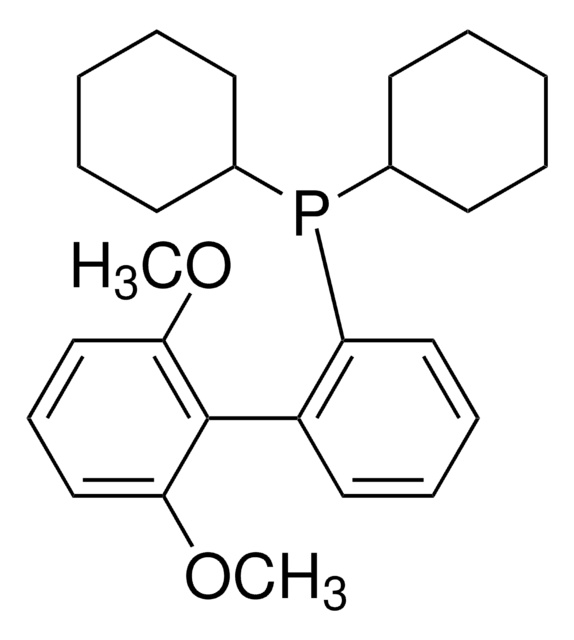
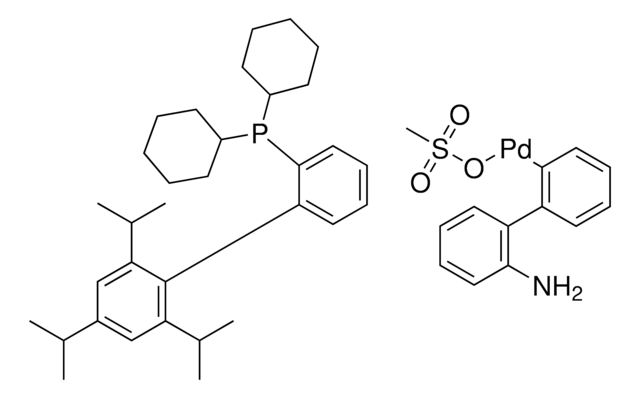
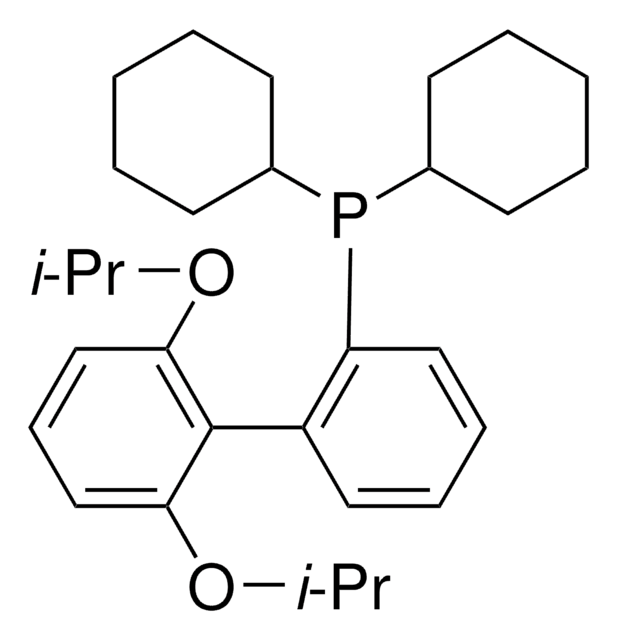
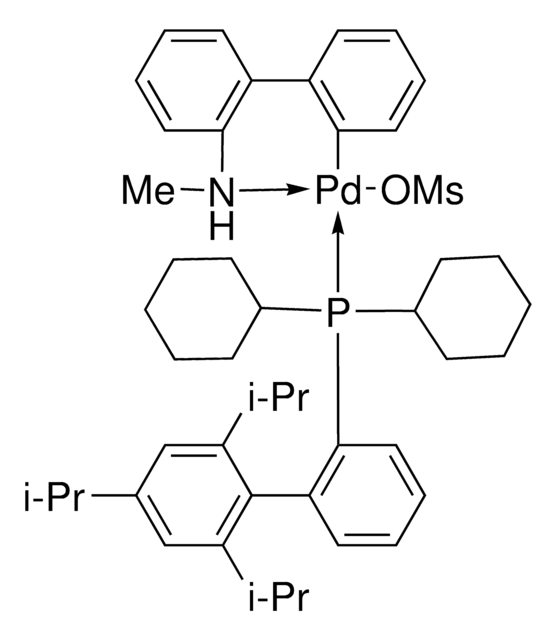
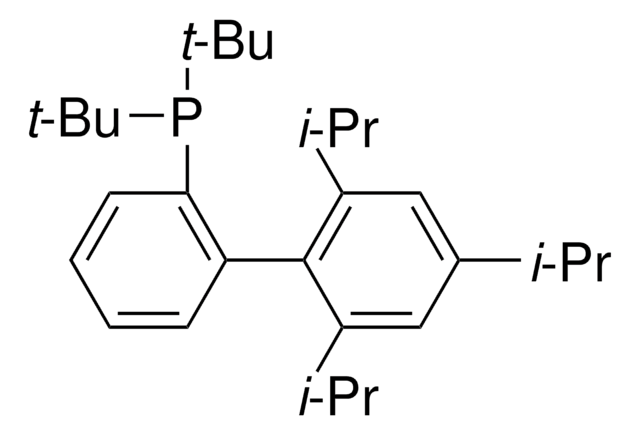
About This Item
추천 제품
Quality Level
분석
98%
반응 적합성
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-C Bond Formation
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Hiyama Coupling
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Stille Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
환경친화적 대안 제품 점수
old score: 2
new score: 1
Find out more about DOZN™ Scoring
환경친화적 대안 제품 특성
Waste Prevention
Atom Economy
Use of Renewable Feedstocks
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
187-190 °C (lit.)
작용기
phosphine
환경친화적 대안 카테고리
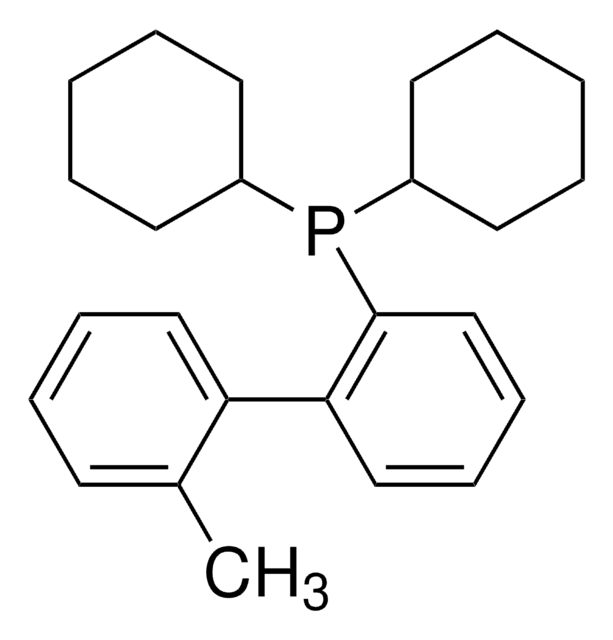
SMILES string
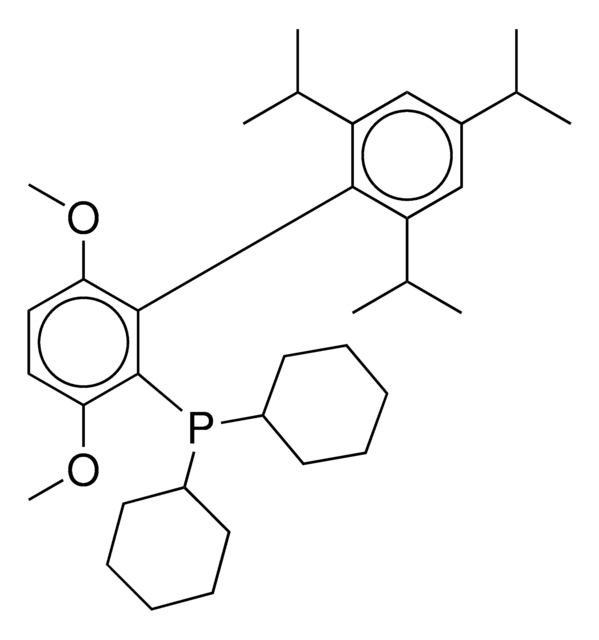
CC(C)C1=CC(C(C)C)=CC(C(C)C)=C1C2=C(P(C3CCCCC3)C4CCCCC4)C=CC=C2
InChI
1S/C33H49P/c1-23(2)26-21-30(24(3)4)33(31(22-26)25(5)6)29-19-13-14-20-32(29)34(27-15-9-7-10-16-27)28-17-11-8-12-18-28/h13-14,19-25,27-28H,7-12,15-18H2,1-6H3
InChI key
UGOMMVLRQDMAQQ-UHFFFAOYSA-N
일반 설명
애플리케이션
Direct annulation of 2-haloanilines to indoles and tryptophans catalyzed by Pd. Synthesis of regioregular polythiophenes.
For small scale and high throughput uses, product is also available as ChemBeads (928364)
On the Way Towards Greener Transition-Metal-Catalyzed Processes as Quantified by E Factors
- Preparation of functionalized benzylic sulfones via palladium-catalyzed Negishi cross-coupling between alkyl sulfones and aryl halides.
- Along with pre-milled palladium(II) acetate as a pre-catalyst for the Stille cross-coupling of aryl chlorides with tributylarylstannanes to form the corresponding biaryl compounds.
- Along with platinum chloride to catalyze the hydrosilylation of terminal arylalkynes with silanes to form functionalized β-(E)-vinylsilanes.
법적 정보
관련 제품
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
문서
A variety of palladium-catalyzed cross-coupling reactions can be run under mild room temperature conditions in water with TPGS- 750-M, using a variety of commercially available palladium complexes and ligands.
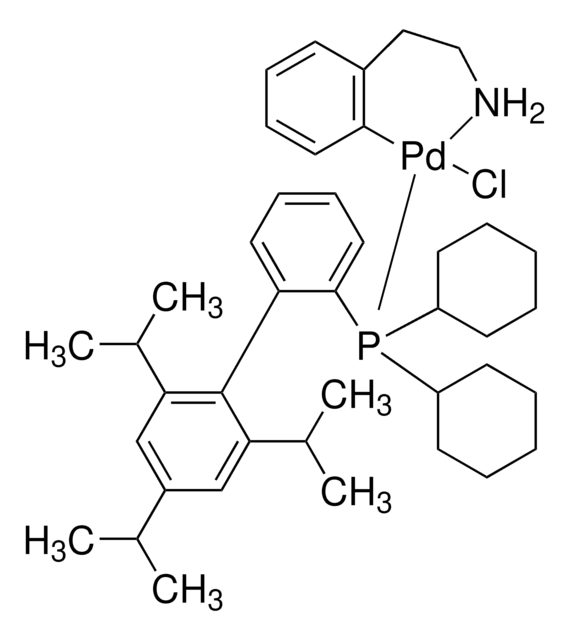
Buchwald Phosphine Ligands
TPGS-750-M, a second generation surfactant, is useful for room temperature, palladium and ruthenium-catalyzed reactions in water. Reactions include the Heck reaction, Suzuki-Miyaura reaction, Sonogashira reaction, Buchwald-Hartwig amination reaction, Negishi reaction, and olefin metathesis.
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.