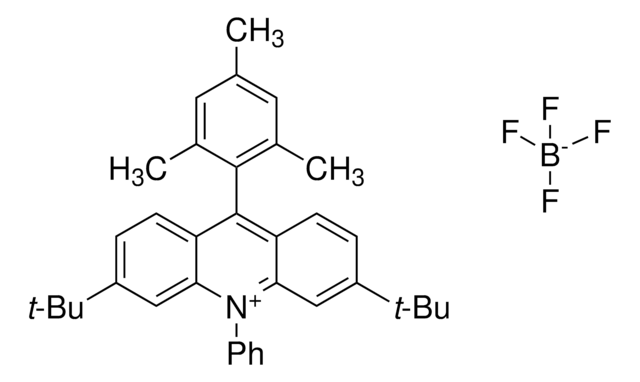
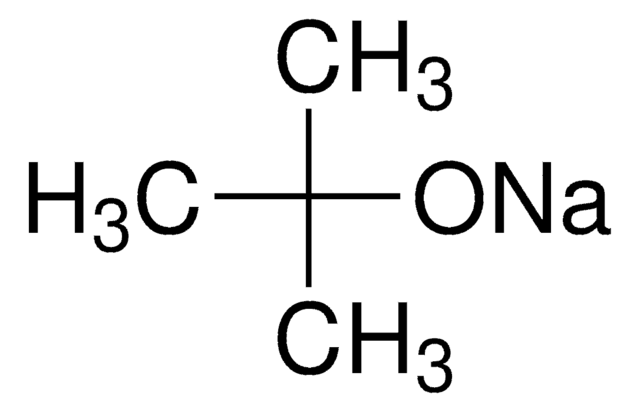
모든 사진(1)
About This Item
실험식(Hill 표기법):
C20H25N
CAS Number:
Molecular Weight:
279.42
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.23
추천 제품
분석
97%
형태
powder
mp
228-233
SMILES string
CC(C)(C)c1ccc2[nH]c3ccc(cc3c2c1)C(C)(C)C
InChI
1S/C20H25N/c1-19(2,3)13-7-9-17-15(11-13)16-12-14(20(4,5)6)8-10-18(16)21-17/h7-12,21H,1-6H3
InChI key
OYFFSPILVQLRQA-UHFFFAOYSA-N
일반 설명
3,6-Di-tert-butylcarbazole is a carbazole based material with hole transporting characteristics. The 3,6-Di-tert-butyl component of the carbazole results in an increase in the glass transition temperature (Tg) of the compound. It can be used in combination with another carbazole to form novel electroluminescent materials.
애플리케이션
3,6-Di-tert-butylcarbazole is mainly used as a monomeric precursor in the syntheses of new carbazole based materials which consist of ethynylphenyl. These materials include 9-(4-bromophenyl)-3,6-di-tert-butylcarbazol and 2-(4-(2-(4-(3,6-di-tert-butyl-9H-carbazol-9-yl)phenyl)ethynyl)benzylidene)malononitrile (PBM) which can be further be used in organic light emitting diodes (OLEDs) and optical switching devices.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Carbazole based hole transporting materials for solid state dye sensitizer solar cells: role of the methoxy groups.
Degbia M, et al.
Polymer International, 63(8), 1387-1393 (2014)
Solvent dependant optical switching in carbazole-based fluorescent nanoparticles.
Adhikari RM, et al.
Langmuir, 25(4), 2402-2406 (2009)
Synthesis and photophysical properties of carbazole-based blue light-emitting dendrimers.
Adhikari RM, et al.
The Journal of Organic Chemistry, 72(13), 4727-4732 (2007)
Bis (carbazolyl) derivatives of pyrene and tetrahydropyrene: synthesis, structures, optical properties, electrochemistry, and electroluminescence.
Kaafarani BR, et al.
Journal of Material Chemistry C, 1(8), 1638-1650 (2013)
Ji Won Yang et al.
Physical chemistry chemical physics : PCCP, 18(45), 31330-31336 (2016-11-09)
Bis(phenylsulfone) was developed as a strong electron acceptor of thermally activated delayed fluorescent emitters. The connection of two electron withdrawing phenylsulfone moieties through meta-position of phenyl produced the bis(phenylsulfone) acceptor and the strong electron acceptor strength of bis(phenylsulfone) enabled preparation
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.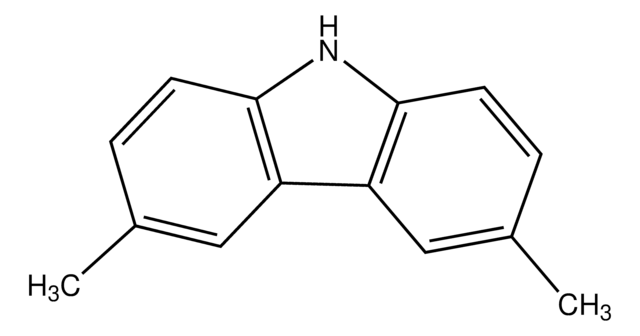

![[9-(4-Bromophenyl)]-3,6-di-tert-butyl-9H-carbazole](/deepweb/assets/sigmaaldrich/product/structures/214/779/819c00e2-ee0a-4166-977c-a7f68002b43d/640/819c00e2-ee0a-4166-977c-a7f68002b43d.png)