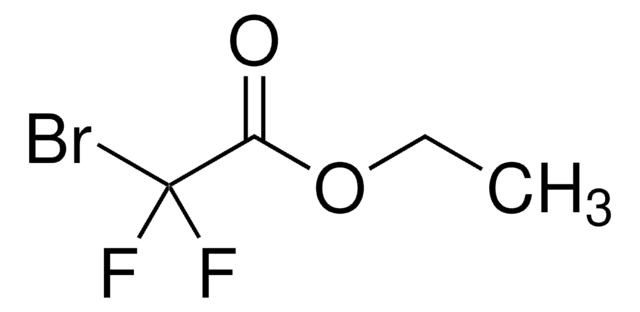
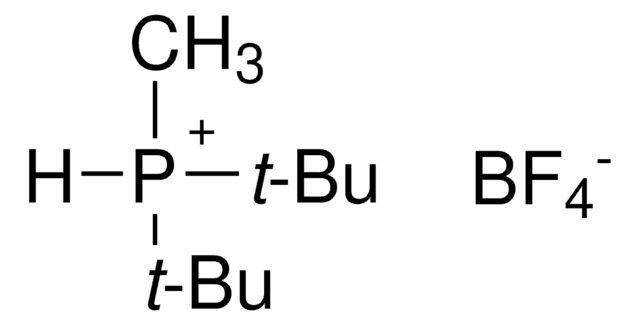추천 제품
Quality Level
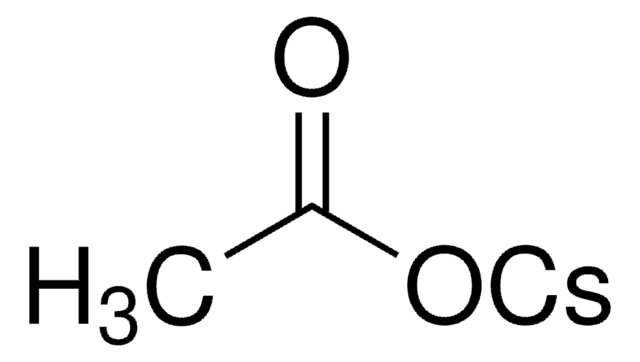
분석
98%
형태
powder
mp
344-348 °C
SMILES string
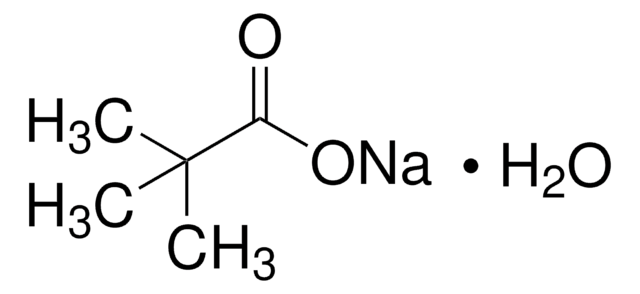
[Cs+].CC(C)(C)C([O-])=O
InChI
1S/C5H10O2.Cs/c1-5(2,3)4(6)7;/h1-3H3,(H,6,7);/q;+1/p-1
InChI key
LGVUAXNPXVXCCW-UHFFFAOYSA-M
일반 설명
Cesium pivalate is an organic base widely used in palladium-catalyzed cross-coupling and carbonylation reactions due to its solubility in organic solvents.
애플리케이션
Cesium pivalate can be used as a base to synthesize:
- Fluoren-9-one derivatives by cyclocarbonylation of o-halobiaryls in the presence of palladium catalyst.
- Fused heterocycles (dihydrobenzofurans and indolines) from o-bromo phenol and aniline precursors via Pd-catalyzed intramolecular coupling of two C(sp3)-H bonds.
- Amides and esters derivatives containing a quaternary β-carbon atom by Pd-catalyzed C-H activation and amino/alkoxycarbonylation reaction.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
M A Campo et al.
Organic letters, 2(23), 3675-3677 (2000-11-14)
The synthesis of various substituted fluoren-9-ones has been accomplished by a novel palladium-catalyzed cyclocarbonylation of o-halobiaryls. The cyclocarbonylation of 4'-substituted-2-iodobiphenyls produces very high yields of 2-substituted fluoren-9-ones bearing either electron-donating or electron-withdrawing substituents. 3'-Substituted 2-iodobiphenyls afford in excellent yields with
Tomáš Čarný et al.
Angewandte Chemie (International ed. in English), 59(43), 18980-18984 (2020-07-22)
The 1,4-palladium shift strategy allows the functionalization of remote C-H bonds that are difficult to reach directly. Reported here is a domino reaction proceeding by C(sp3 )-H activation, 1,4-palladium shift, and amino- or alkoxycarbonylation, which generates a variety of amides
Zubaoyi Yi et al.
The Journal of organic chemistry, 82(13), 6946-6957 (2017-06-16)
Pd-catalyzed arylation or benzylation of nitroazoles using aryl sulfonates or benzyl acetates is described. Electronically varied aryl tosylates and mesylates, as well as benzyl acetates, afford the arylated and benzylated products. Arylation of nitrobenzene is also reported. The relative rate
Aditya L Gottumukkala et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(11), 3091-3095 (2011-02-10)
Ace of base: A catalytic system is presented that, solely by choice of the base, selectively switches between conjugate addition and the Mizoroki-Heck reaction of aryl halides with Michael acceptors (see scheme; R, R' = alkyl, aryl). For conjugate addition
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
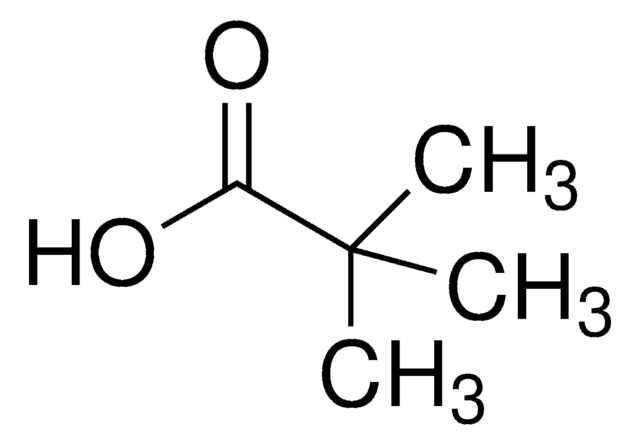

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)