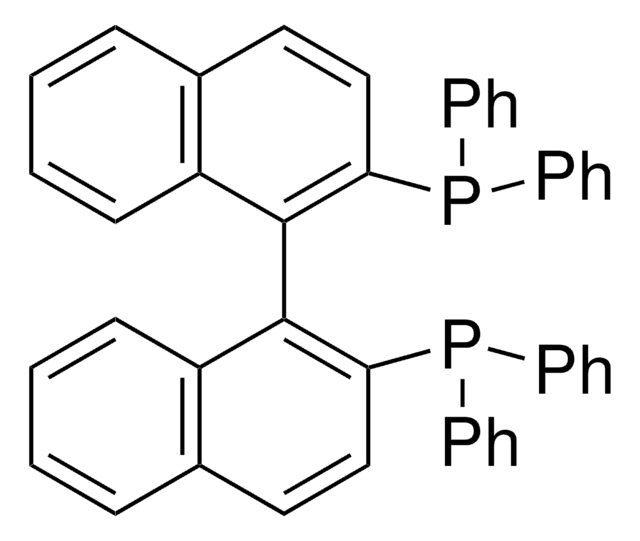
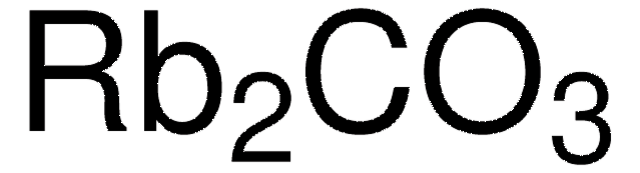추천 제품
일반 설명
애플리케이션
법적 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Repr. 2 - STOT RE 2 Oral
표적 기관
Kidney,Adrenal gland,Testes
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
문서
JosiPhos CyPF-tBu and palladium give catalyst for alkoxylation of activated heteroaryl halides with primary, secondary, and tertiary alcohols
A solid and bench-stable alternative to sulfuryl fluoride gas has been developed, 4-(Acetylamino)phenyl]imidodisulfuryl difluoride (ASIF). ASIF is a shelf-stable, crystallilne reagent for the installation of the valuable SO2F functional group.
Professor Aran (Claremont University, USA) thoroughly discusses the engineering of graphene based materials through careful functionalization of graphene oxide, a solution processable form of graphene.
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.