632961
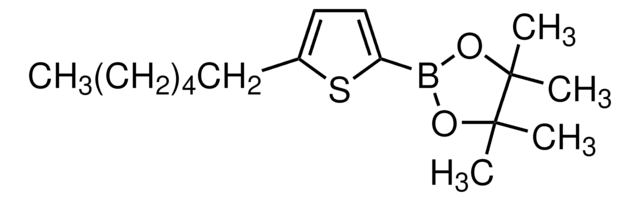
5′-Hexyl-2,2′-bithiophene-5-boronic acid pinacol ester
97%
동의어(들):
2-(5′-Hexyl-2,2′-bithien-5-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-[5′-hexyl-2,2′-bithien-5-yl]-1,3,2-dioxaborolane, 5′-N-Hexyl-2,2′-bithiophene-5-boronic acid pinacol ester, 5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-5′-N-hexyl-2,2′-bithiophene, 5-Hexyl-5′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2,2′-bithiophene
About This Item
추천 제품
Quality Level
분석
97%
양식
solid
mp
36-40 °C (lit.)
SMILES string
CCCCCCc1ccc(s1)-c2ccc(s2)B3OC(C)(C)C(C)(C)O3
InChI
1S/C20H29BO2S2/c1-6-7-8-9-10-15-11-12-16(24-15)17-13-14-18(25-17)21-22-19(2,3)20(4,5)23-21/h11-14H,6-10H2,1-5H3
InChI key
XTTRNSNHDCYSEL-UHFFFAOYSA-N
애플리케이션
- Suzuki-Miyaura cross-coupling reactions and shape-shifting in contorted dibenzotetrathienocoronenes
- Oligothiophene self-assembly induction into fibers with tunable shape and function
- Stille coupling and p-conjugated packing structure and hole mobility of bithiophene-bithiazole copolymers with alkyl-thiophene side chains
Reagent used in Preparation of
- Solution-processed ambipolar field-effect transistor
- Light harvesting small molecules for use in solution-processed small molecule bulk heterojunction solar cell devices
- Light-emitting diode (OLED) materials
- Unsymmetric substituted benzothiadiazole-containing vinyl monomers for RAFT polymerization
- Pd-catalyzed condensations and synthesis of isoindigo-based oligothiophenes for molecuar bulk heterojunction solar cells
- Thiophene-benzothiadiazole based donor-acceptor-donor materials
문서
Oligothiophenes are important organic electronic materials which can be produced using synthetic intermediates and Suzuki coupling.
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.