모든 사진(1)
About This Item
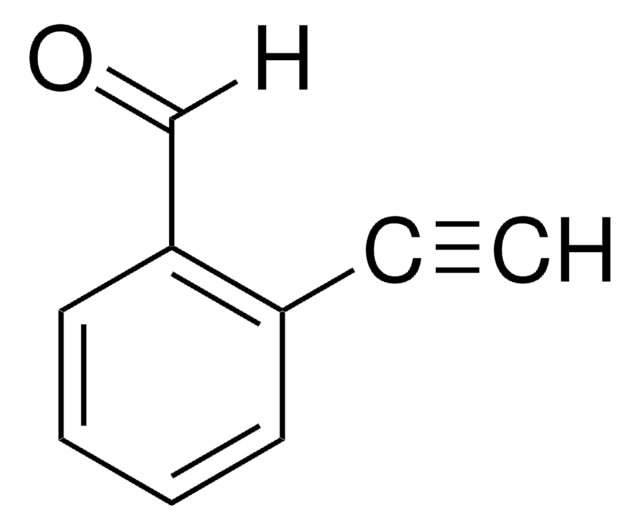
Linear Formula:
IC6H4CHO
CAS Number:
Molecular Weight:
232.02
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
mp
36-39 °C (lit.)
작용기
aldehyde
iodo
저장 온도
2-8°C
SMILES string
Ic1ccccc1C=O
InChI
1S/C7H5IO/c8-7-4-2-1-3-6(7)5-9/h1-5H
InChI key
WWKKTHALZAYYAI-UHFFFAOYSA-N
일반 설명
2-Iodobenzaldehyde (o-iodobenzaldehyde) is a 2-halobenzaldehyde derivative. Its crystals belong to the orthorhombic crystal system and P212121 space group.
애플리케이션
2-Iodobenzaldehyde may be used as a reactant in the synthesis of the following heterocycles:
- 2,3-diaryl-1-indenones
- indolo[1,2-a]quinazolines
- Baylis-Hillman (BH) adducts
- 5-phenylindazolo[3,2-b]quinazolin-7(5H)-one
- 4-(3-iodophenyl)-2,2:6,2-terpyridine
- fluoren-9-one
- 2-formyl-3′-methoxybiphenyl
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Copper (I)-Catalyzed Synthesis of 5-Arylindazolo [3, 2-b] quinazolin-7 (5 H)-one via Ullmann-Type Reaction
Chen DS, et al.
The Journal of Organic Chemistry, 78(11), 5700-5704 (2013)
A simple copper-catalyzed two-step one-pot synthesis of indolo [1, 2-a] quinazoline.
Li C, et al.
Beilstein Journal of Organic Chemistry, 10(1), 2441-2447 (2014)
Ring-Closing Olefin Metathesis of 2, 2'-Divinylbiphenyls: A Novel and General Approach to Phenanthrenes.
Iuliano A, et al.
Organic Letters, 6(21), 3711-3714 (2004)
2-Iodobenzaldehyde.
Betz R and Klufers P.
Acta Crystallographica Section E, Structure Reports Online, 63(12), o4879-o4879 (2007)
Synthesis of indanones via intramolecular Heck reaction of Baylis-Hillman adducts of 2-iodobenzaldehyde.
Park JB, et al.
Bull. Korean Chem. Soc., 25(6), 927-930 (2004)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.