418633
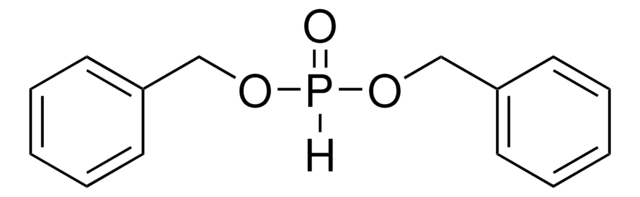
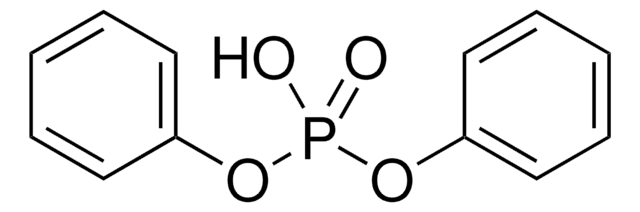
Tetrabenzyl pyrophosphate
98%
동의어(들):
Pyrophosphoric acid tetrabenzyl ester, Tetrabenzyl diphosphate
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
(C6H5CH2O)2P(O)OP(O)(OCH2C6H5)2
CAS Number:
Molecular Weight:
538.47
Beilstein:
2068292
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
mp
63-66 °C (lit.)
작용기
phenyl
phosphate
저장 온도
−20°C
SMILES string
O=P(OCc1ccccc1)(OCc2ccccc2)OP(=O)(OCc3ccccc3)OCc4ccccc4
InChI
1S/C28H28O7P2/c29-36(31-21-25-13-5-1-6-14-25,32-22-26-15-7-2-8-16-26)35-37(30,33-23-27-17-9-3-10-18-27)34-24-28-19-11-4-12-20-28/h1-20H,21-24H2
InChI key
NSBNXCZCLRBQTA-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Tetrabenzyl pyrophosphate may be employed for the following studies:
- Fabrication of Pb2+-selective membrane electrodes.
- Preparation of synthetic nucleotides, phosphates of the 3,6-dideoxyhexoses.
- As phosphorylating reagent for the synthesis of Und-PP-Bac (undecaprenyl pyrophosphate = Und-PP; Bac = unusual sugar bacillosamine).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Solvolysis of Tetrabenzyl Pyrophosphate. Catalysis by Imidazole1
Blakeley R, et al.
Journal of the American Chemical Society, 88(1), 112-119 (1966)
Journal of the Chemical Society. Perkin Transactions 1, 729-729 (1992)
D Xu et al.
Talanta, 51(2), 365-371 (2008-10-31)
Tetrabenzyl pyrophosphate and diphenylphosphinic anhydride, with two phosphoryl groups (PO) as ligating sites, can be used as novel ionophores to make Pb(2+)-selective membrane electrodes. A good result was obtained with tetrabenzyl pyrophosphate, and the electrode based on this ionophore and
N S Utkina et al.
Bioorganicheskaia khimiia, 15(10), 1375-1383 (1989-10-01)
Interaction of lithium alcoholates of 2,4-di-O-benzoates of paratose and abequose with tetrabenzyl pyrophosphate gave alpha-phosphates of the 3,6-dideoxyhexoses, further converted into the corresponding cytidine-5'-diphosphate derivatives. These synthetic nucleotides were shown to participate in the biosynthesis of the O-specific polysaccharides for
Eranthie Weerapana et al.
Journal of the American Chemical Society, 127(40), 13766-13767 (2005-10-06)
The chemical synthesis and biological activity of undecaprenyl pyrophosphate bacillosamine (Und-PP-Bac), an obligatory intermediate in the asparagine-linked glycosylation pathway of Campylobacter jejuni, are reported. The key transformation involves the coupling of bacillosamine phosphate and undecaprenyl phosphate. The synthetic Und-PP-Bac can
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.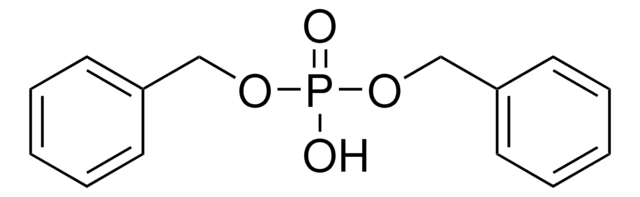



![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)