모든 사진(2)
About This Item
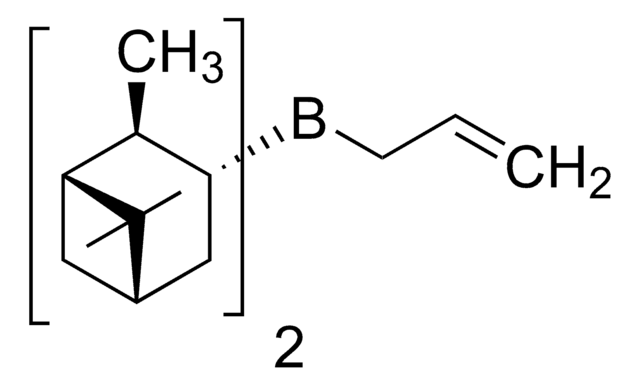
실험식(Hill 표기법):
C18H31B
CAS Number:
Molecular Weight:
258.25
MDL number:
UNSPSC 코드:
12352005
PubChem Substance ID:
추천 제품
Quality Level
분석
97%
광학 활성
[α]21/D -22°, c = 12 in THF
bp
>55 °C (lit.)
density
0.947 g/mL at 25 °C (lit.)
SMILES string
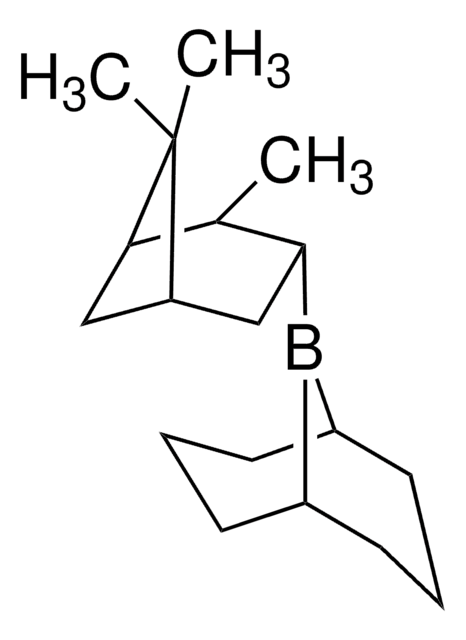
C[C@H]1[C@@H](CC2CC1C2(C)C)B3C4CCCC3CCC4
InChI
1S/C18H31B/c1-12-16-10-13(18(16,2)3)11-17(12)19-14-6-4-7-15(19)9-5-8-14/h12-17H,4-11H2,1-3H3/t12-,13+,14-,15+,16-,17-/m1/s1
InChI key
VCDGSBJCRYTLNU-PHPOFCCKSA-N
일반 설명
R-Alpine-Borane® is a chiral reducing agent, synthesized from (+)-α-pinene via hydroboration.
애플리케이션
R-Alpine-Borane® may be used in the preparation of (22R)-hydroxy-23-acetylenic steroids with high stereoselectivity.
Reagent for the asymmetric reduction of a variety of prochiral ketones.
법적 정보
Alpine-Borane is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Pyr. Liq. 1
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
Ramachandran, P.V. et al.
Tetrahedron Asymmetry, 4, 2399-2399 (1993)
Stereocontrolled synthesis of 22-hydroxy-23-acetylenic steroids, key intermediates in steroid side chain construction. Observation of a directive effect by an a-chiral site during asymmetric reduction with-B-3-pinanyl-9-BBN (Alpine-Borane).
Midland MM and Kwon YC.
Tetrahedron Letters, 25(52), 5981-5984 (1984)
Diisopinocampheylchloroborane, a remarkably efficient chiral reducing agent for aromatic prochiral ketones.
Chandrasekharan J, et al.
The Journal of Organic Chemistry, 50(25), 5446-5448 (1985)
Matteson DS
Stereodirected Synthesis with Organoboranes, 346-347 (2012)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.