추천 제품
Quality Level
분석
99%
양식
powder
mp
167-170 °C (lit.)
작용기
amine
SMILES string
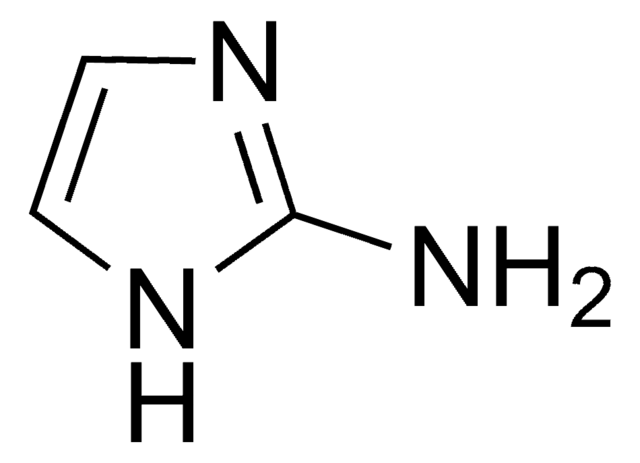
Cl[H].NC(=N)n1cccn1
InChI
1S/C4H6N4.ClH/c5-4(6)8-3-1-2-7-8;/h1-3H,(H3,5,6);1H
InChI key
RBZRMBCLZMEYEH-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
1H-Pyrazole-1-carboxamidine hydrochloride, a pyrazole derivative, is a heterocyclic compound. It is widely used in drug syntheses studies. Pyrazole ring forms the main core of various nonsteroidal anti-inflammatory drugs (NSAIDs) and antihypertensive drugs.
애플리케이션
1H-Pyrazole-1-carboxamidine hydrochloride may be used in the following studies:
- Preparation of guanidylated hollow fiber membranes.
- Guanylation of amines and in peptide synthesis.
- Synthesis of bis-guanidinium-cholesterol derivatives.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Sens. 1 - STOT RE 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Hironori Izawa et al.
Biomolecules, 9(7) (2019-07-10)
In order to synthesize a promising material for developing a novel peptide/protein delivery system, guanidinylation of chitooligosaccharides with 1-amidinopyrazole hydrochloride was investigated herein. The production of guanidinylated chitooligosaccharides was demonstrated by infrared spectroscopy (IR), nuclear magnetic resonance (NMR), and elemental
1H-Pyrazole-1-carboxamidine hydrochloride an attractive reagent for guanylation of amines and its application to peptide synthesis.
Bernatowicz MS, et al.
The Journal of Organic Chemistry, 57(8), 2497-2502 (1992)
Guanidinium-cholesterol cationic lipids: efficient vectors for the transfection of eukaryotic cells.
J P Vigneron et al.
Proceedings of the National Academy of Sciences of the United States of America, 93(18), 9682-9686 (1996-09-03)
Two cationic lipids, bis-guanidinium-spermidine-cholesterol (BGSC) and bis-guanidinium-trencholesterol (BGTC)-cholesterol derivatives bearing two guanidinium groups-have been synthesized and tested as artificial vectors for gene transfer. They combine the membrane compatible features of the cholesterol subunit and the favorable structural and high pKa
Xiao Zhang et al.
ACS applied materials & interfaces, 12(14), 16088-16096 (2020-03-17)
Supramolecular hydrogels have great potential as biomaterials for tissue engineering applications or vehicles for delivering therapeutic agents. Herein, a self-healing and pro-osteogenic hydrogel system is developed based on the self-assembly of laponite nanosheets and guanidinylated chitosan, where laponite works as
Ekaterina K Ogurtsova et al.
Natural product communications, 10(7), 1171-1173 (2015-09-29)
The guanidine alkaloids, dihydropulchranin A (2), prepared from pulchranin A from the sponge Monanchora pulchra, and hexadecylguanidine (3), a synthetic analog of pulchranins, were studied for their TRPV channel-regulating activities. Compound 2 was active as an inhibitor of rTRPV1 and
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 402516-10G | 4061832993638 |
| 402516-50G | 4061831985900 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.