모든 사진(2)
About This Item
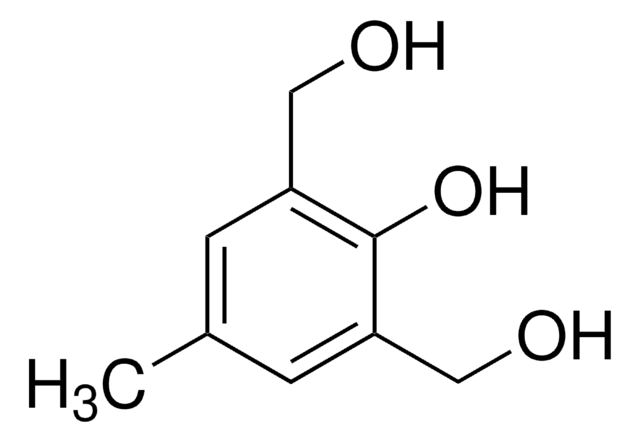
Linear Formula:
O2NC6H2(OCH3)2CH2Br
CAS Number:
Molecular Weight:
276.08
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
97%
mp
131-133 °C (lit.)
작용기
bromo
nitro
SMILES string
COc1cc(CBr)c(cc1OC)[N+]([O-])=O
InChI
1S/C9H10BrNO4/c1-14-8-3-6(5-10)7(11(12)13)4-9(8)15-2/h3-4H,5H2,1-2H3
InChI key
UEKFEYNZISYRRH-UHFFFAOYSA-N
일반 설명
4,5-Dimethoxy-2-nitrobenzyl bromide (DMNBB, 1-(Bromomethyl)-4,5-dimethoxy-2-nitrobenzene) is a 4,5-dimethoxy-2-nitrobenzyl derivative. 4,5-Dimethoxy-2-nitrobenzyl (DMNB) group of DMNBB is used as a photolabile protecting group in caging technology to develop pro-drugs. Synthesis of 1-(bromomethyl)-4,5-dimethoxy-2-nitrobenzene by using 6-nitroveratraldehyde as starting reagent has been reported.
애플리케이션
4,5-Dimethoxy-2-nitrobenzyl bromide (DMNB bromide, 6-nitroveratryl bromide) is suitable reagent used in the synthesis of N-(4,5-dimethoxy-2-nitrobenzyl)vanillylamine which forms caged vanilloid. It may be used in the synthesis of the following:
- 4-(4′,5′-dimethoxy-2-nitrobenzyloxy)benzaldehyde, a DMNB-caged aldehyde
- N-[4-[(4,5-dimethoxy-2-nitrobenzyl)oxy]-3-methoxybenzyl]acetamide
- caged derivative of pyridostatin ([C]-PDS)
- photosensitive polyimide (PI-DMNB)
- caged β-ecdysone
- 4-tert-butyldimethylsilyloxy-1-(2-deoxy-3,5-di-O-toluoyl-β-D-ribofuranosyl)-2-(6-nitroveratrylthio)-1H-benzimidazole, an intermediate in synthesis of phosphoramidite bearing 4-hydroxy-2-mercaptobenzimidazole (SBNV) nucleobase
- alkylation of dihydrofluorescein
- 4-(4′,5′-Dimethoxy-2′-nitrobenzyloxy)benzaldehyde
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Surface functionalization of PEEK films using photochemical routes.
Henneuse-Boxus C, et al.
Eur. Polymer J., 37(1), 9-18 (2001)
Pierre Murat et al.
Chemical communications (Cambridge, England), 49(76), 8453-8455 (2013-08-21)
The use of a caged G-quadruplex ligand allows for transcriptional control of quadruplex-containing genes using UV light as an external trigger. An important oncogene, SRC, involved in the initiation and proliferation of epithelial tumours is shown to be significantly downregulated
G Marriott et al.
Biochemistry, 35(10), 3170-3174 (1996-03-12)
An understanding of the molecular mechanism of muscle contraction will require a complete description of the kinetics of the myosin motor in vitro and in vivo. To this end chemical relaxation studies employing light-directed generation of ATP from caged ATP
Light-triggered strand exchange reaction using the change in the hydrogen bonding pattern of a nucleobase analogue.
Morihiro K, et al.
Chemical Science, 5(2), 744-750 (2014)
Synthesis and Characterizations of Positive-Working Photosensitive Polyimides Having 4, 5-Dimethoxy-o-Nitrobenzyl Side Group.
Ryu S, et al.
Bull. Korean Chem. Soc., 29(9), 1689-1689 (2008)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.